13.Sulphuryl chloride, SO2Cl2, reacts with H2O to give mixture of H2So4 and HCL. Aqueous solution of 1 mol SO2Cl2 will be neutralised by ?. (1) 3 moles of NaOH (3) Both (1)

Sulphuryl chloride SO2Cl2, reacts with H2O to give mixture of H2SO4 and HCl. Aqueous solution of 1mole of SO2Cl2 will be neutralized by:a)3 moles of NaOHb)2 moles of Ca(OH)2c)Bothd)None of these Correct

53. Sul CU (3) CIO Sulphuryl chloride SO.CI, reacts with H,O to (4) MnO give mixture of H.SO, and HCL. Aq. solution 1 of 1 mol so.Cl, will be neutralised by: (1)
16. So2cl2 reacts with H2o gives H2so4 and Hcl determine volume of 0.2M Ba(oH)2 required to neutralised 25ml of 0.1 M so2cl2
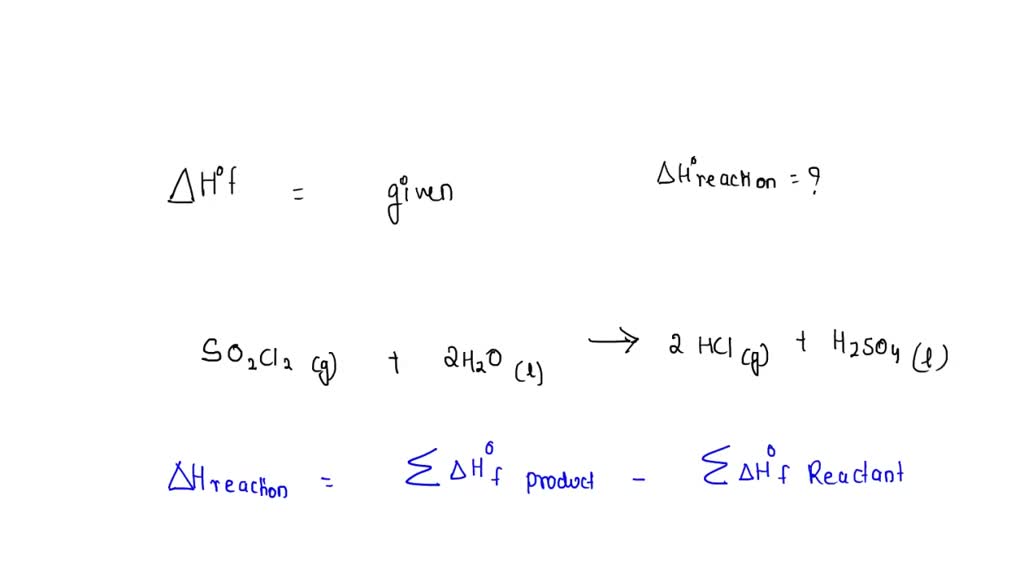
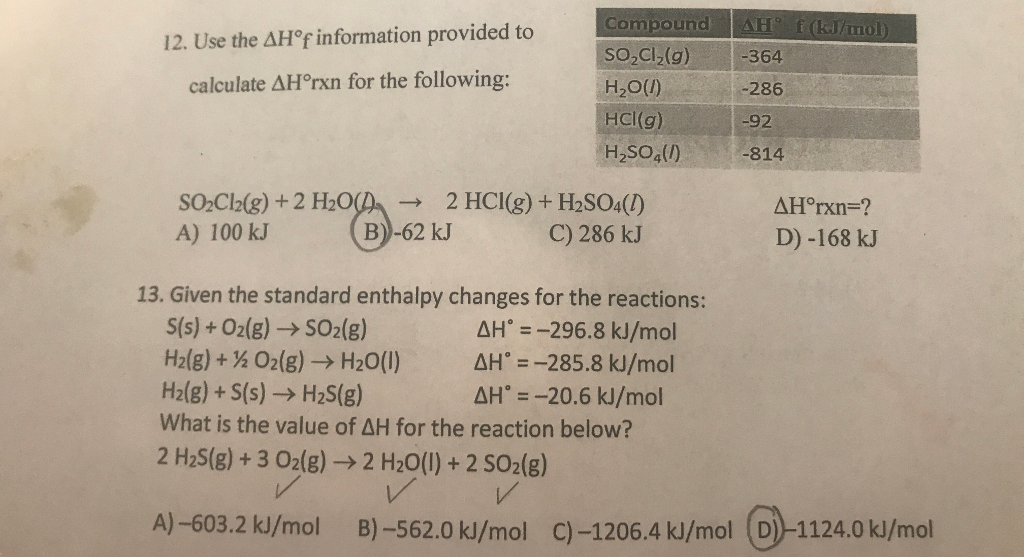
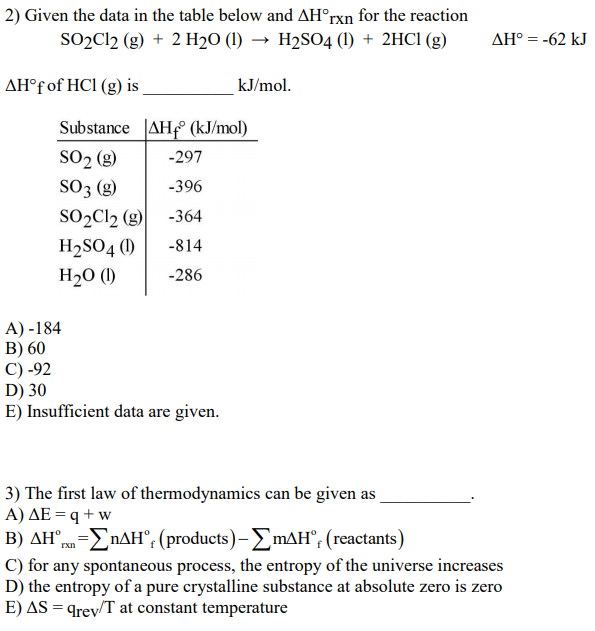
SOLVED: Use the ΔH°f information provided to calculate ΔH°rxn for the following: ΔH°f (kJ/mol) SO2Cl2(g) + 2 H2O(l) â†' 2 HCl(g) + H2SO4(l) ΔH°rxn = ? SO2Cl2(g) -364 H2O(l) -286 HCl(g) -92

1 mole of SO2Cl2 is dissolved in water and Ca(OH)2 is added to neutralise acidic solution. The number of moles of Ca(OH)2 required is ______.
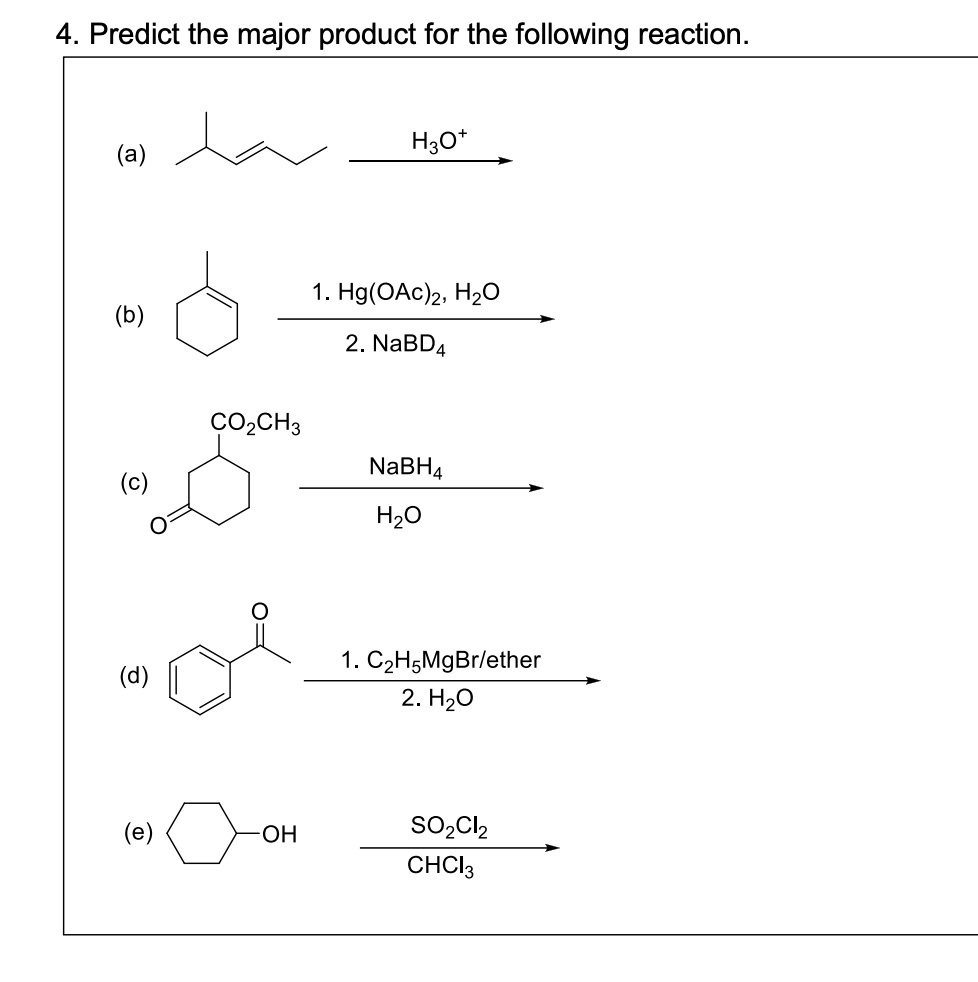
SOLVED: 4. Predict the major product for the following reaction H3O a 1.Hg(OAc)2,H2O (b) 2.NaBD4 CO2CH3 NaBH4 H2O 1.C2H5MgBr/ether 2.H2O SO2Cl2 CHCI3 (e) OH
SO2Cl2 on reaction with excess of water results into acidic mixture SO2Cl2 + 2H2O → H2SO4 + 2HCl - Sarthaks eConnect | Largest Online Education Community
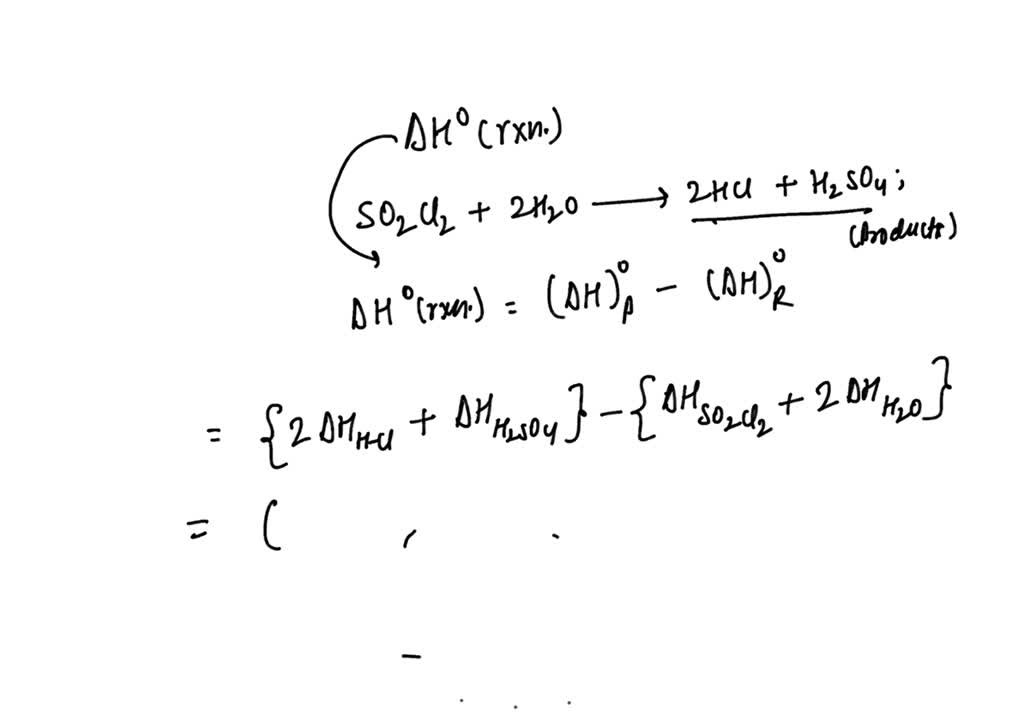
SOLVED: Calculate the ΔH°rxn for the following reaction: SO2Cl2 + 2H2O â†' 2HCl + H2SO4, using the enthalpy of formations. ΔH°f Given: SO2Cl2 = -364 kJ/mol H2O = -286 kJ/mol HCl = -

19. Complete the following equations: i) PA+ SO2Cl2 -- ii) XeF6 + H2O --------- iii) Cu + HNO3 (Conc.) ---