Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A

Kinetic Study of Ni and NiO Reactions Pertinent to the Earth's Upper Atmosphere | The Journal of Physical Chemistry A

IJMS | Free Full-Text | One Step Synthesis of NiO Nanoparticles via Solid-State Thermal Decomposition at Low-Temperature of Novel Aqua(2,9-dimethyl-1,10-phenanthroline)NiCl2 Complex
Charge-storage mechanism of highly defective NiO nanostructures on carbon nanofibers in electrochemical supercapacitors - Nanoscale (RSC Publishing)

The Calotropis procera Transformed Green NiO and Fe‐NiO Nanoparticles for Diaryl Pyrimidinones Synthesis in Hydrotropic Medium at Room Temperature - Shinde - 2018 - ChemistrySelect - Wiley Online Library
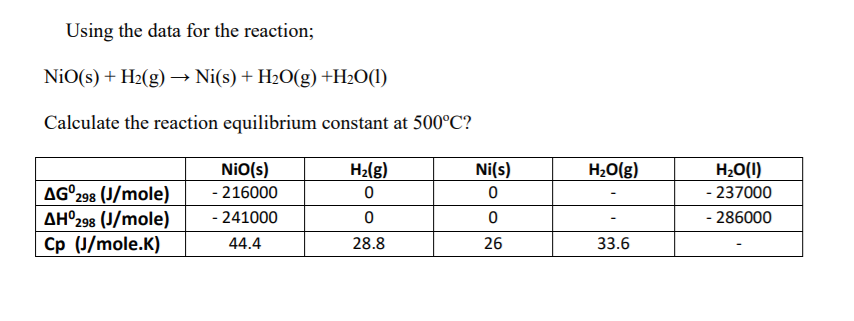
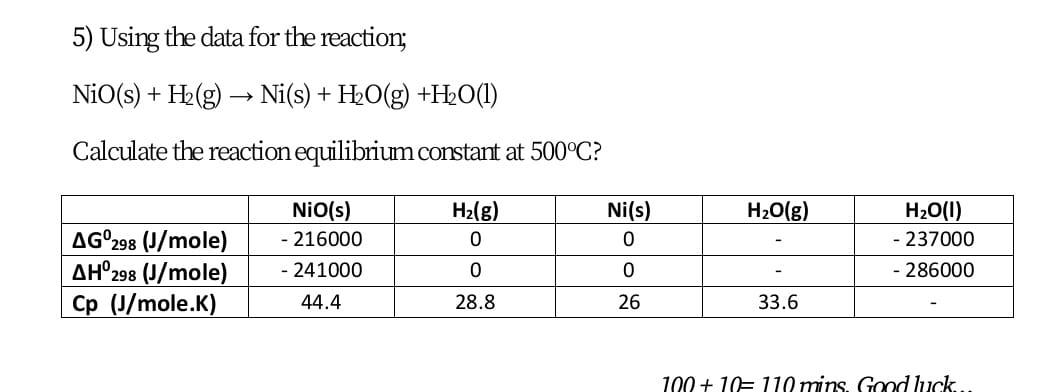
![SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2] SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2]](https://cdn.numerade.com/project-universal/previews/f52234cd-9af7-477c-b7a1-a1075ed3eb39.gif)
SOLVED: Which is the correct equilibrium constant expression for the following reaction? NiO(s) + H2(g) Ni(aq) + H2O(g) a) Kc = [H2]/[H2O] b) Kc = [Ni][H2O]/[Ni2O3] [H2] c) Kc = [Ni] [H2O]/[H2]

Identify from the following reactions the reactants that undergo oxidation and reduction.NiO+H2⟶Ni+H2O

Calculated physical parameters such as energy (E) as eV, total dipole... | Download Scientific Diagram

Construction of NiO/H2Ti3O7 nanotube composite and its photocatalytic conversion feature for ethyl mercaptan | Applied Physics A

Wet etch rate of NiO in 1:4 HNO3:H2O as a function of solution temperature. | Download Scientific Diagram

a) ELF on Se/γ-NiOOH, γ-NiOOH and NiO/α-FeOOH models. (b), (c) The DOS... | Download Scientific Diagram