Using this balanced equation: 2 NaOH + H2SO4 —> H2O + Na2SO4 How many grams of sodium sulfate will be - brainly.com

PDF) Thermodynamics of crystallization of sodium sulfate decahydrate in H2O–NaCl–Na2SO4: application to Na2SO4·10H2O-based latent heat storage materials | Roland Solimando - Academia.edu

Phase Equilibrium of the Ternary System Na2SO3–Na2SO4–H2O at 293.15, 313.15, and 353.15 K | Journal of Chemical & Engineering Data
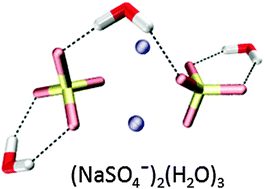
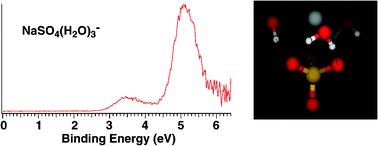
Probing the microsolvation of a quaternary ion complex: gas phase vibrational spectroscopy of (NaSO4−)2(H2O)n=0–6, 8 - Physical Chemistry Chemical Physics (RSC Publishing)
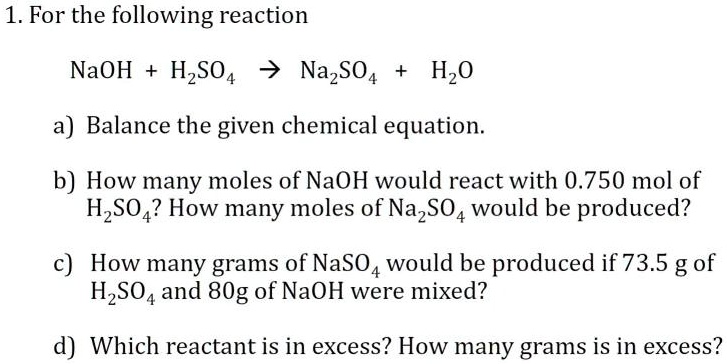
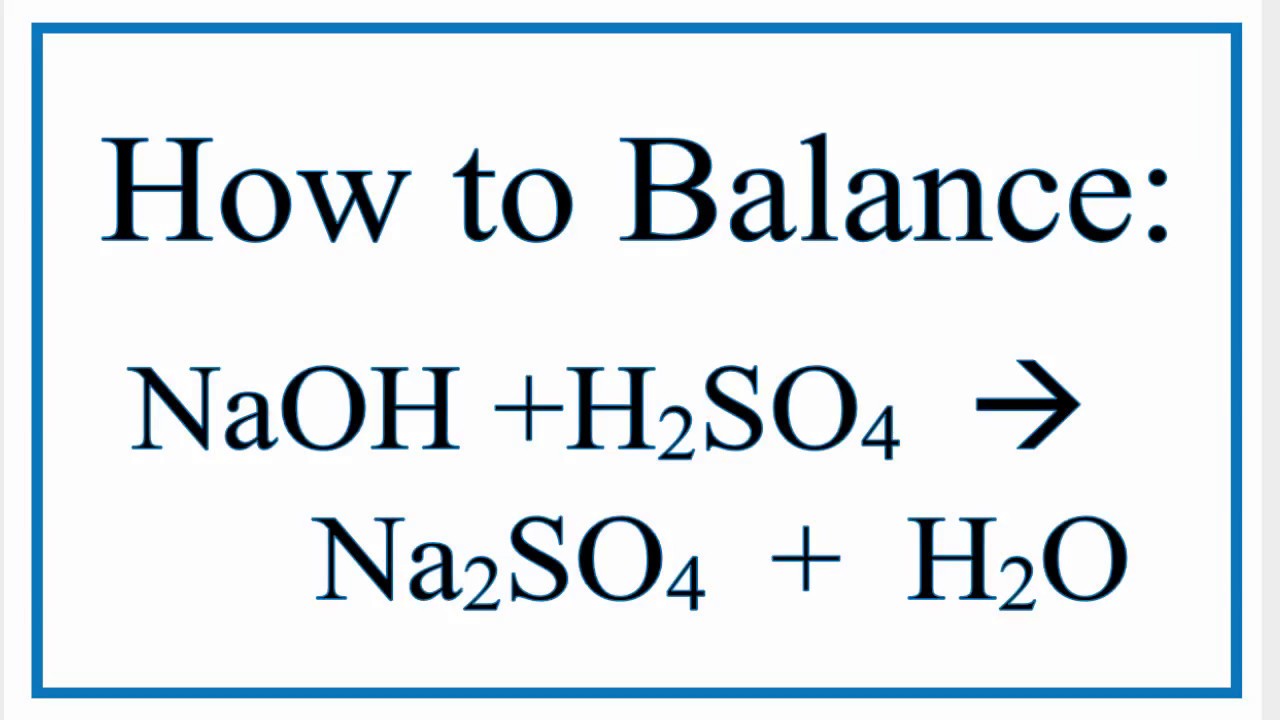
SOLVED: Texts: 1. For the following reaction: NaOH + HSO4 -> NaSO4 + H2O a) Balance the given chemical equation. b) How many moles of NaOH would react with 0.750 mol of

H Balance the following reactions NaOH + H2SO4 → Na2SO4+H2O 7 CuSO4 + NaOH → Cu(OH)2 + Na2SO4 Fe + H2O → Fe3O4+H2 4. NH4Cl + Ca(OH)2 CaCl2 + H2O + NH3 6. Fe + Cl2 → FeCl3 3. Fe

SOLVED: Which reaction represents an acid-base neutralization reaction? Hint: How do you identify acids and bases? O 2 NaOH + MgSO4 NaSO4 + Mg(OH)2 O HNO + KOH KNO3 + H2O O













![SODIUM SULFATE, HYDRATE - Optional[FTIR] - Spectrum - SpectraBase SODIUM SULFATE, HYDRATE - Optional[FTIR] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/CNSMv1IsNzL/structure.png?h=300&w=382)