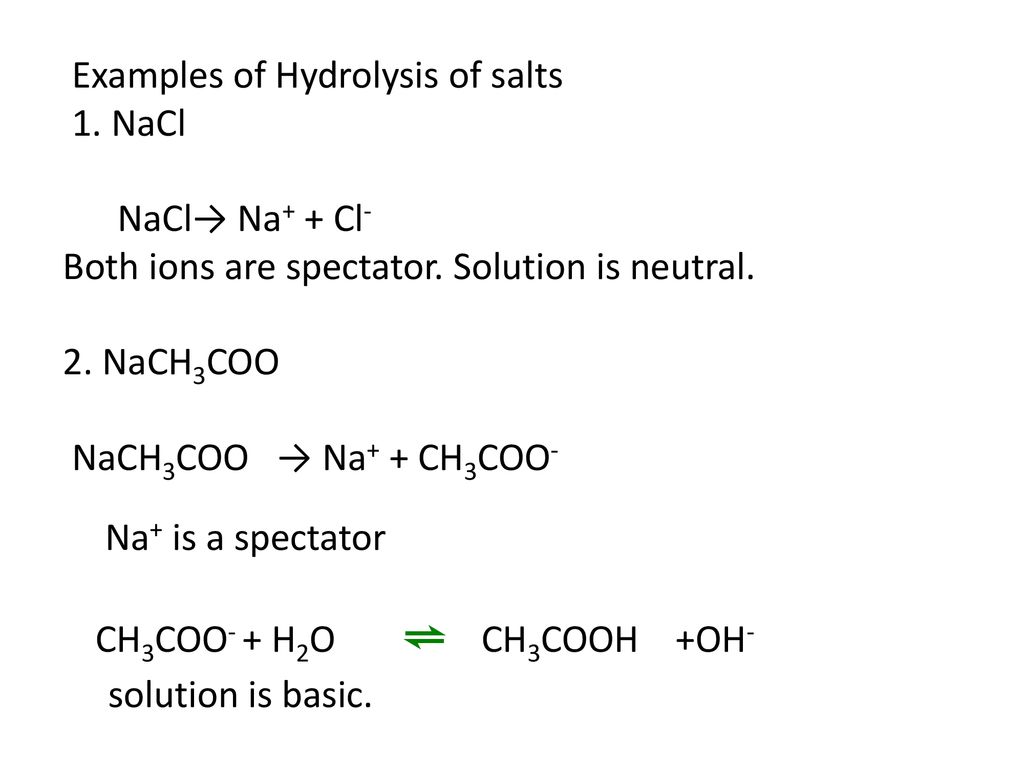
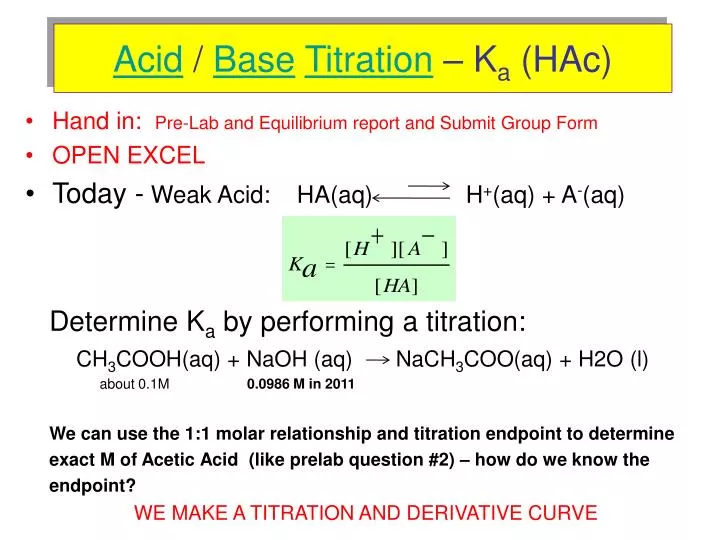
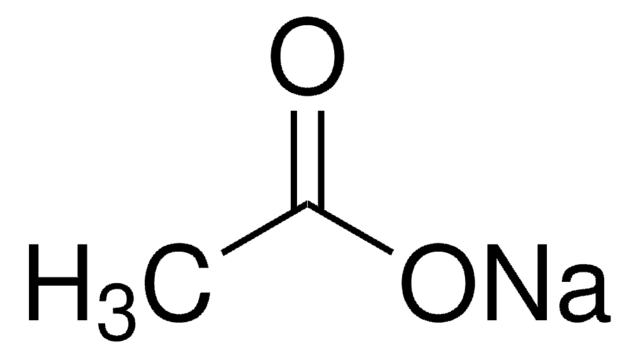
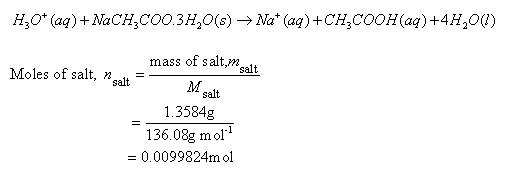Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.

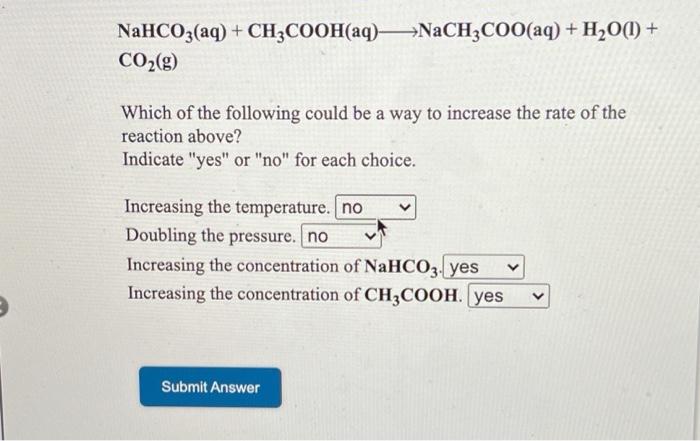
SOLVED: NaHCO3(aq) + CH3COOH(aq) â†' CO2(g) + NaCH3COO(aq) + H2O(l) Which of the following could be a way to increase the rate of the reaction above? Indicate "yes" or "no" for each

Sodium acetate hydrate, Puratronic™, 99.9985% (metals basis), Thermo Scientific Chemicals | Fisher Scientific

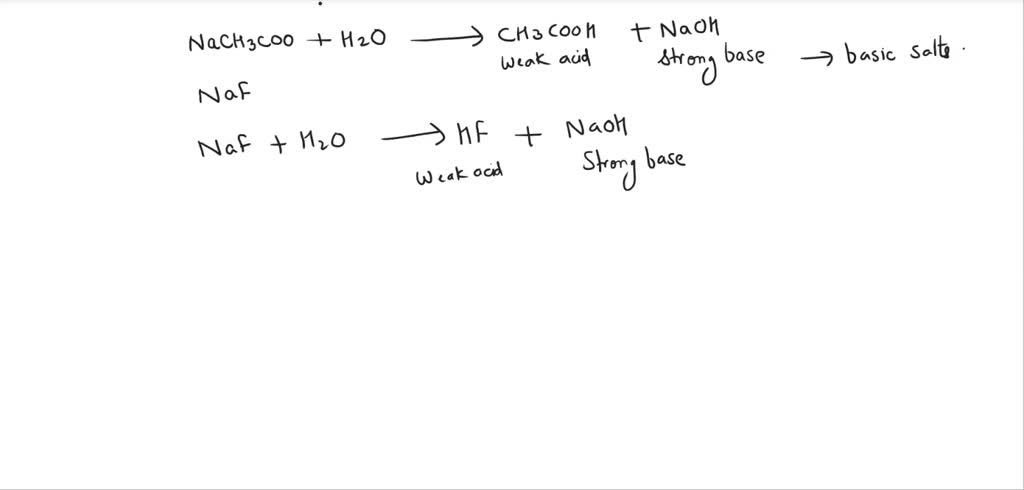
SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow

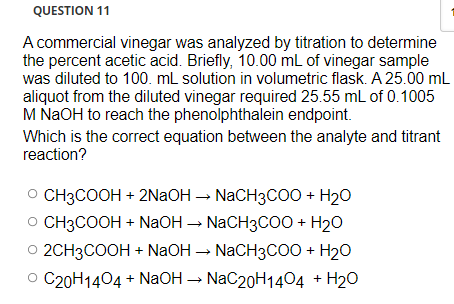
NaOH + X ----> NaCH3COO + H2O What is X in this reaction? A-NH4OH B-H3PO4 C-H2CO3 D-CH3COOH - brainly.com
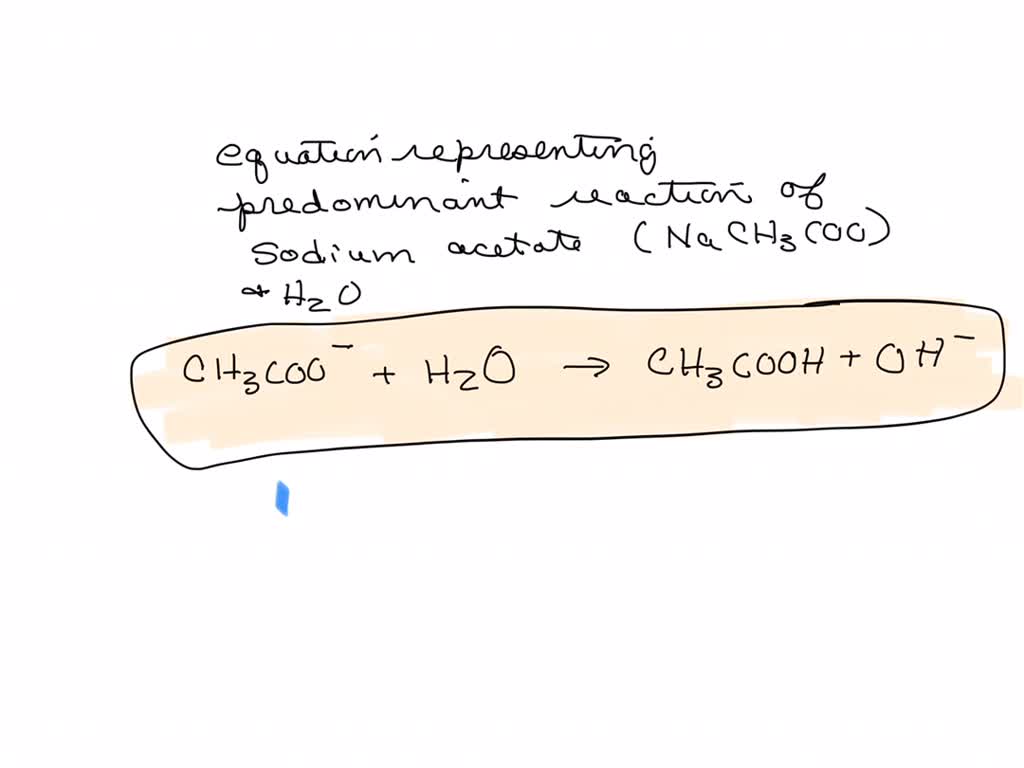
SOLVED: The equation representing the predominant reaction of sodium acetate, NaCH3COO, with water is: CH3COO- + H2O ⇌ CH3COOH + OH- CH3COO- + H2O ⇌ CH3COOH + OH- CH3COOH + H2O ⇌

For sodium acetate solution in water, the given equilibrium reaction occur:CH 3 COO aq + H 2 O l hydrolysis ⇌ CH 3 COOH aq + OH aqWhich of the following describes