Influence of Common Anions on the Coordination of Metal Cations in Polyalcohols - Teichert - 2019 - European Journal of Inorganic Chemistry - Wiley Online Library

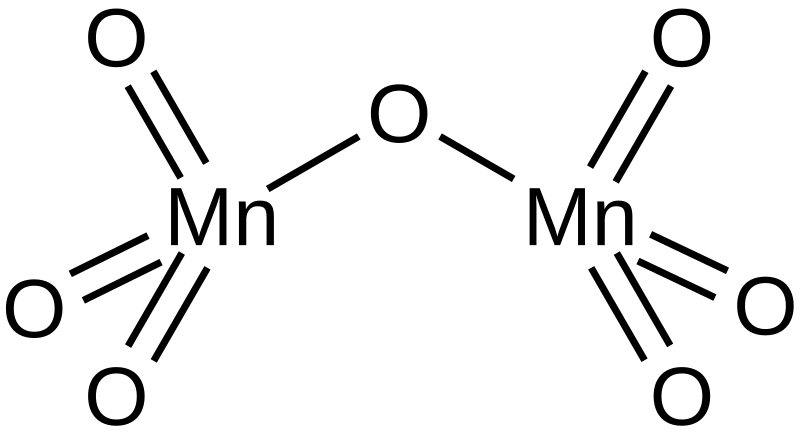
Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega

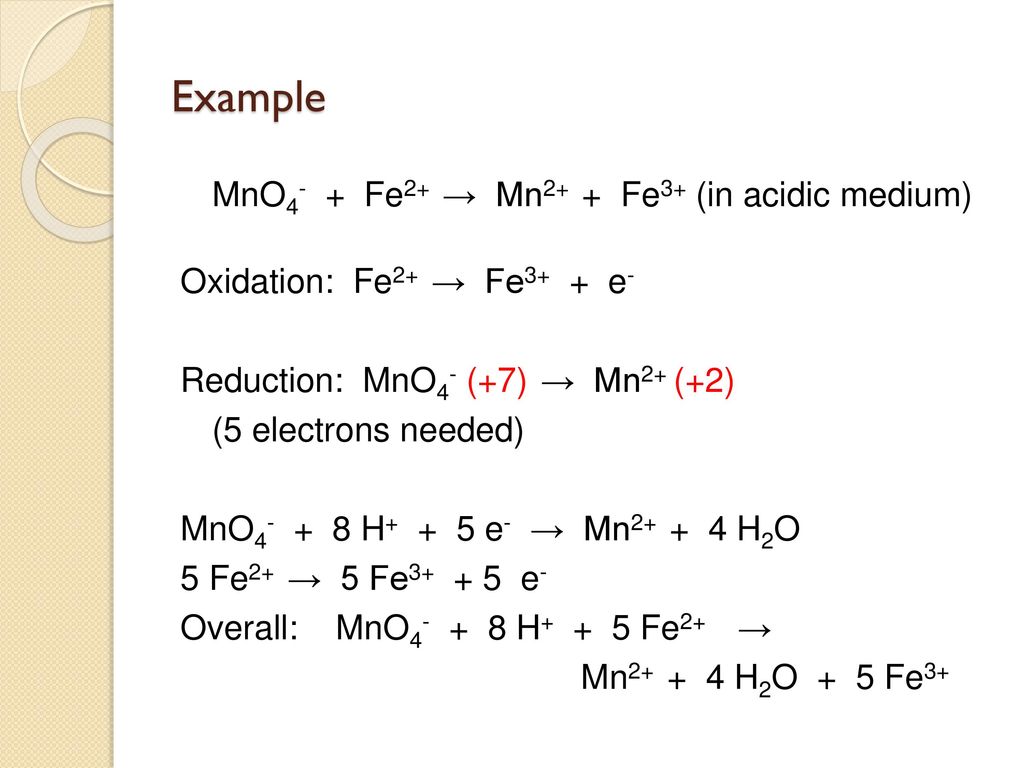
Rare and Nonexistent Nitrosyls: Periodic Trends and Relativistic Effects in Ruthenium and Osmium Porphyrin-Based {MNO}7 Complexes | ACS Omega

Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method | ACS Omega
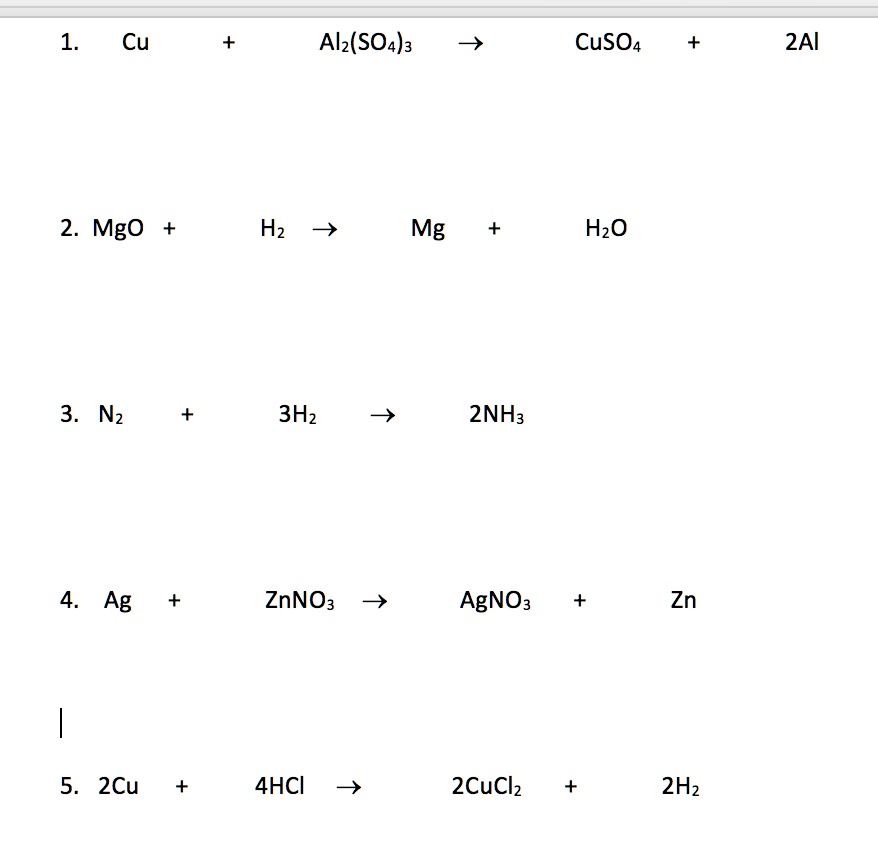
SOLVED: 1. Cu + Al2(SO4)3 â†' CuSO4 + 2Al 2. MgO + H2O â†' Mg + H2O 3. N2 + 3H2 â†' 2NH3 4. Ag + Zn(NO3)2 â†' AgNO3 + Zn 5. 2Cu + 4HCl â†' 2CuCl2 + 2H2
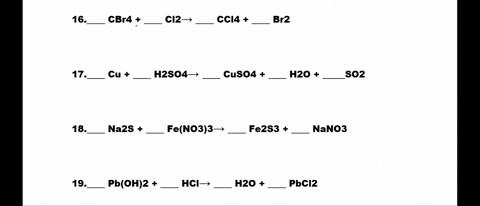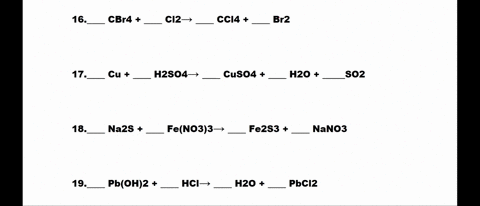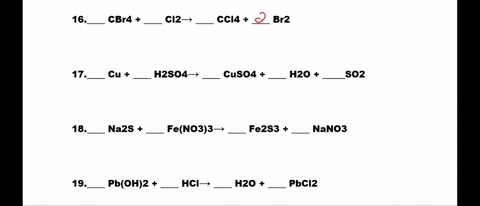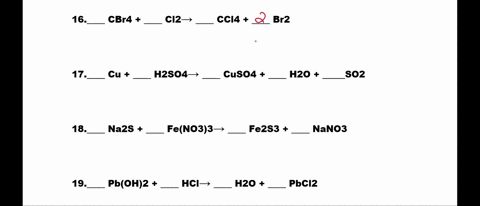
SOLVED: 1. N2 + H2 â†' NH3 2. CH4 + O2 â†' CO2 + H2O 3. P4 + O2 â†' P2O3 4. H2 + NO â†' N2 + H2O 5. Na +

Novel carbohydrate-substituted cyclopentadienyls of titanium, molybdenum, manganese and iron - ScienceDirect