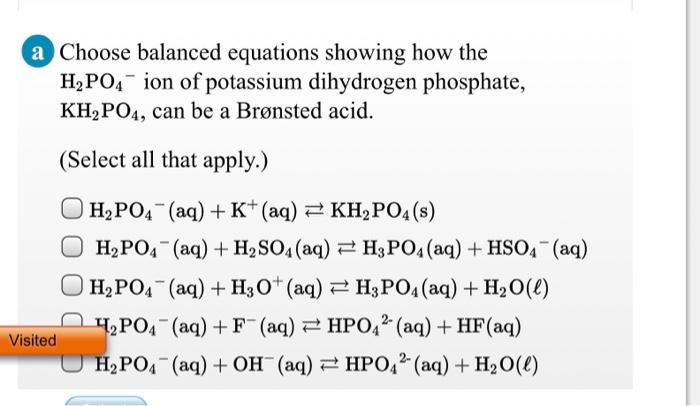
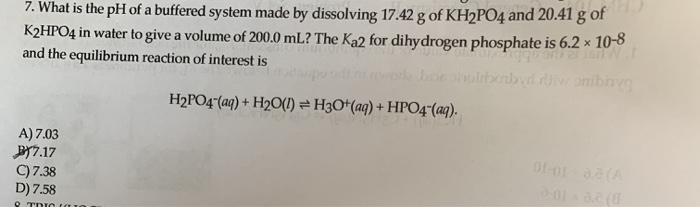Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram

A 0.492 g of KH_2PO_4 is titrated against a solution of 0.112 M NaOH. The volume of the base required to do this is 25.6 mL. The reaction involved is, KH_2PO_4 +

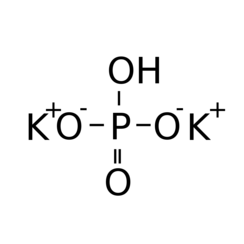
SRL Potassium Dihydrogen Orthophosphate for molecular biology, 99.5% 500Gm, CAS 7778-77-0, Molecular Formula : KH2PO4, Storage : Room Temperature, Shelf Life : 60 Months for laboratory use only : Amazon.in: Industrial & Scientific

Equilibrium phase diagram of the ternary system KH2PO4 + KNO3 + H2O at... | Download Scientific Diagram
![FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub](https://images.squarespace-cdn.com/content/v1/5f02d28f35d64d2a5022eeb1/1611724367429-9L5VAR6883R3CB69ME9Q/20.png)
FilSciHub Ed - CHEMISTRY MODULE] CHEMICAL REACTIONS & CHEMICAL EQUATIONS [ANSWER KEY] — Filipino Science Hub

Jual POTASSIUM DIHIDROGEN PHOSPHATE ANHIDRAT ( KH2PO4.H2O ) PRO ANALISA 10 GRAM MERCK BEST SELLER | Shopee Indonesia
![SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA] SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA]](https://cdn.numerade.com/ask_images/84c1fd22ef3046d0a647ee2adee981f8.jpg)
SOLVED: Describe stepwise how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using KPO4·H2O (f.w. 230.28) and KH2PO4 (f.w. 174.18). [A-] pH = pKa + log [HA]

OneClass: Spectrophotometric analysis of phosphate can be performed by the following procedure: A. KH...

KH2PO4 crystallisation from potassium chloride and ammonium dihydrogen phosphate – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.


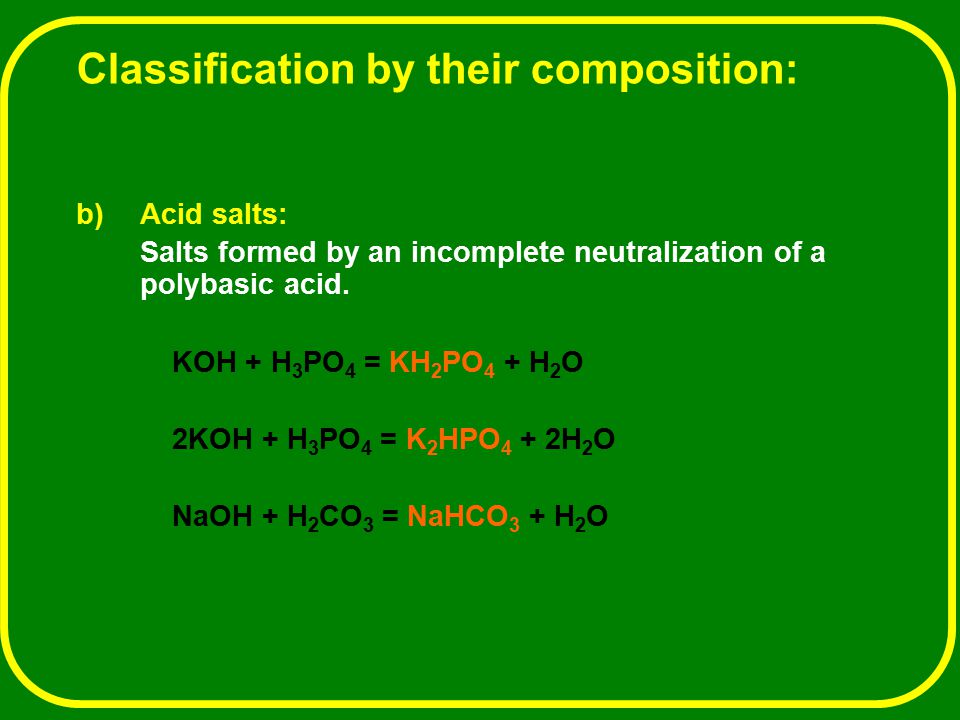

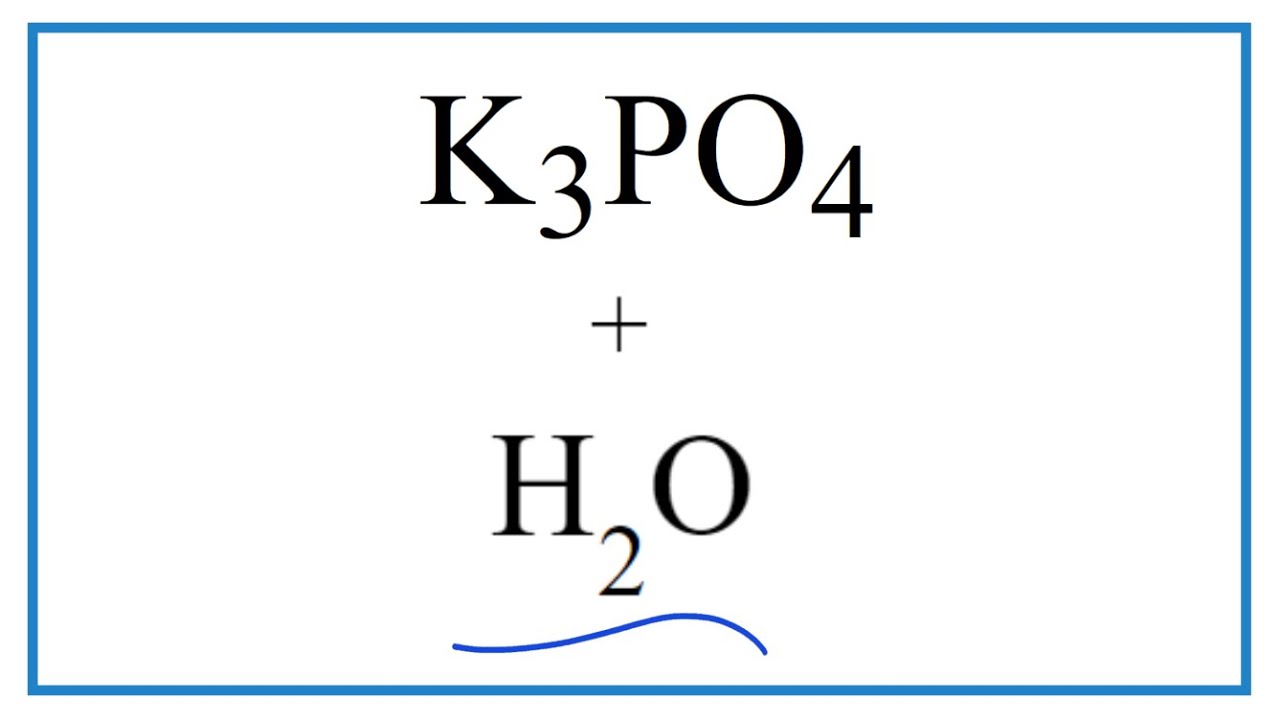

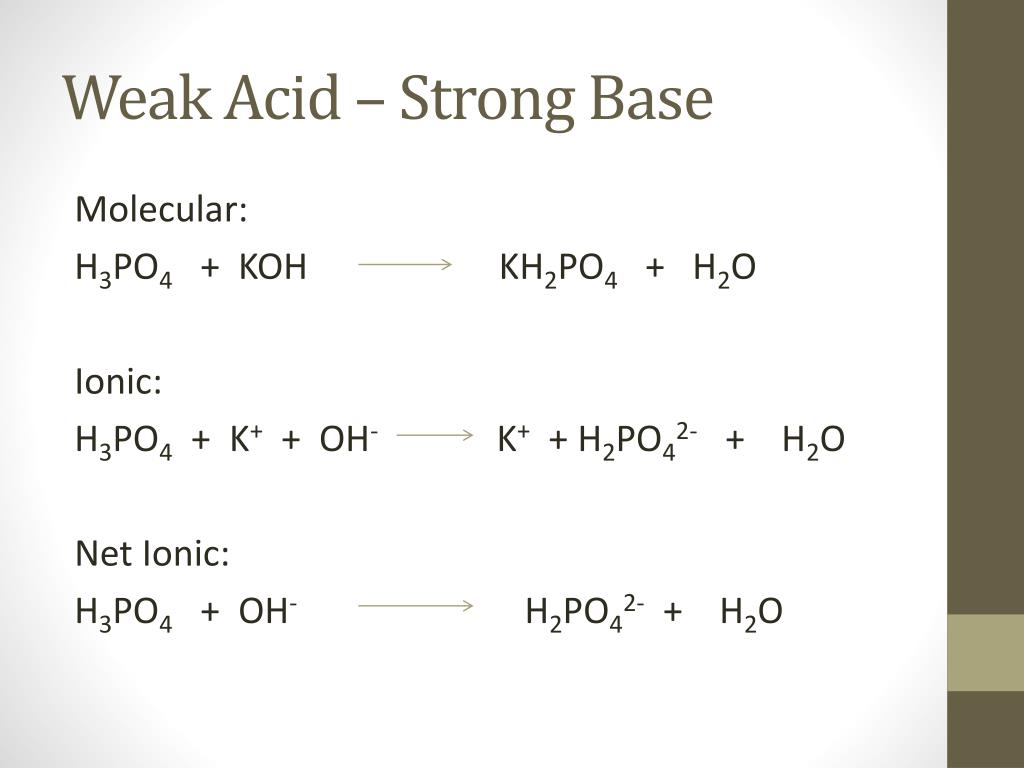



![ANSWERED] c 3 136 A 0 5 g sample of KH PO4 is titra... - Physical Chemistry - Kunduz ANSWERED] c 3 136 A 0 5 g sample of KH PO4 is titra... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20200703122613132777-1655732.jpg)