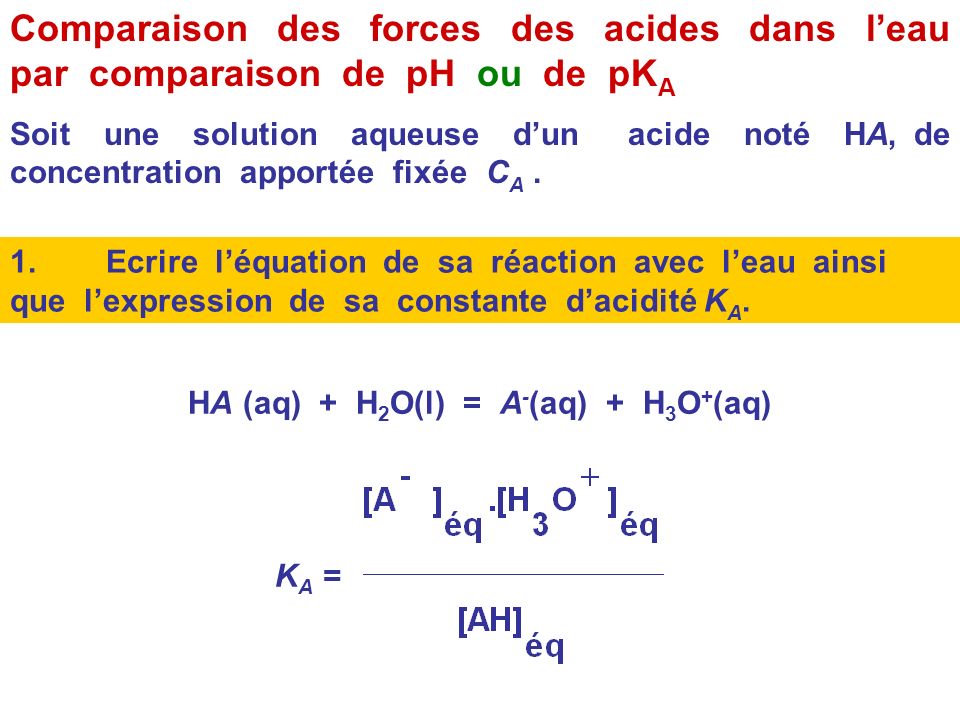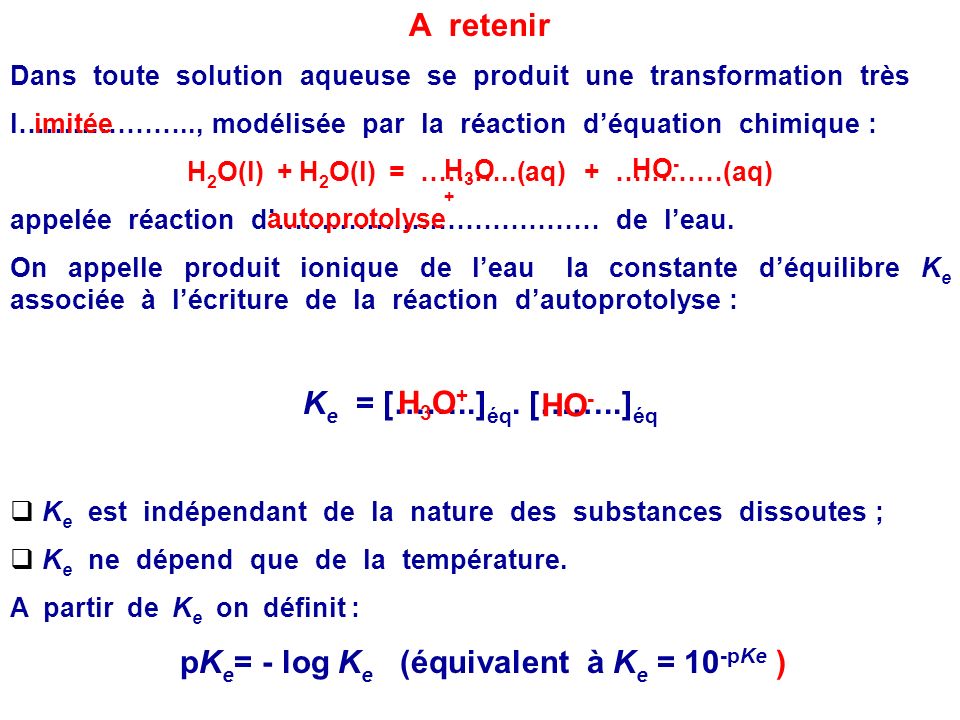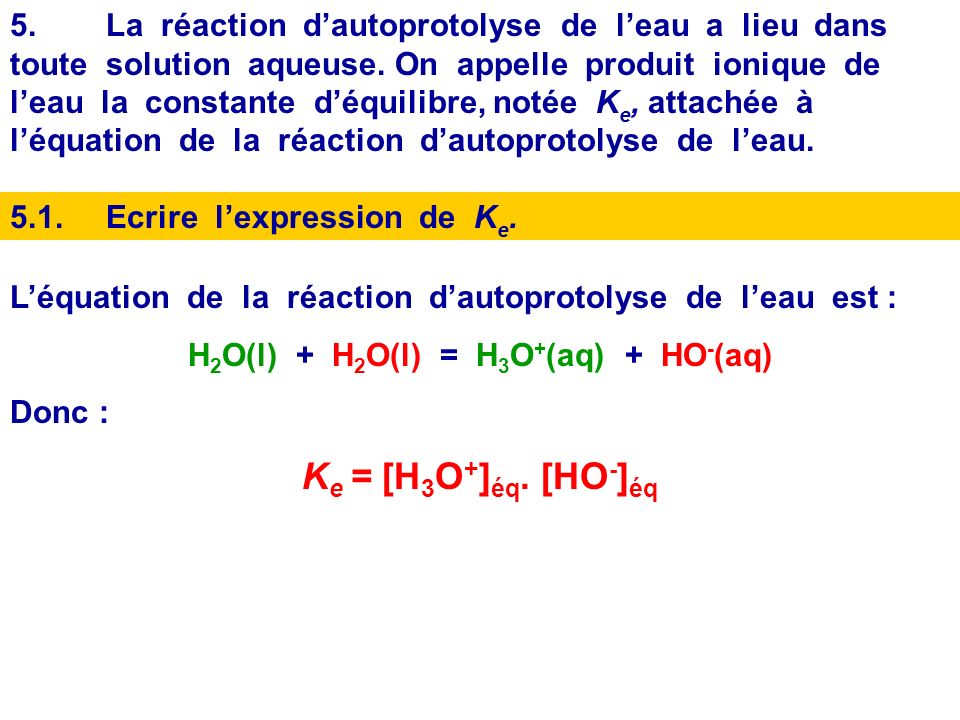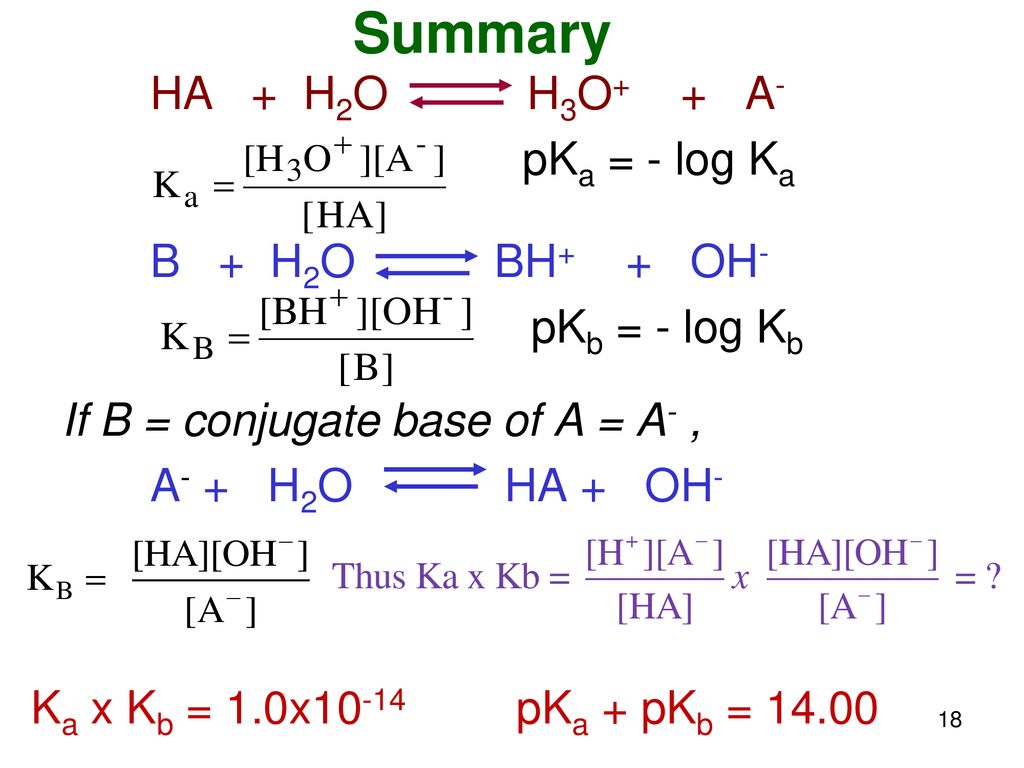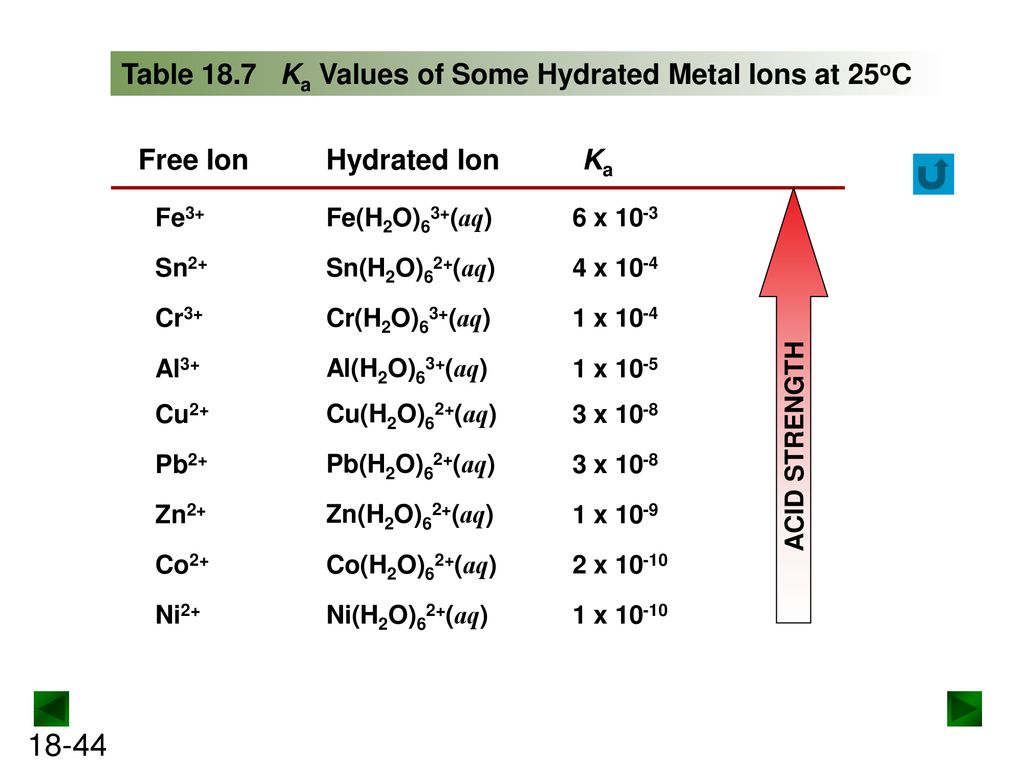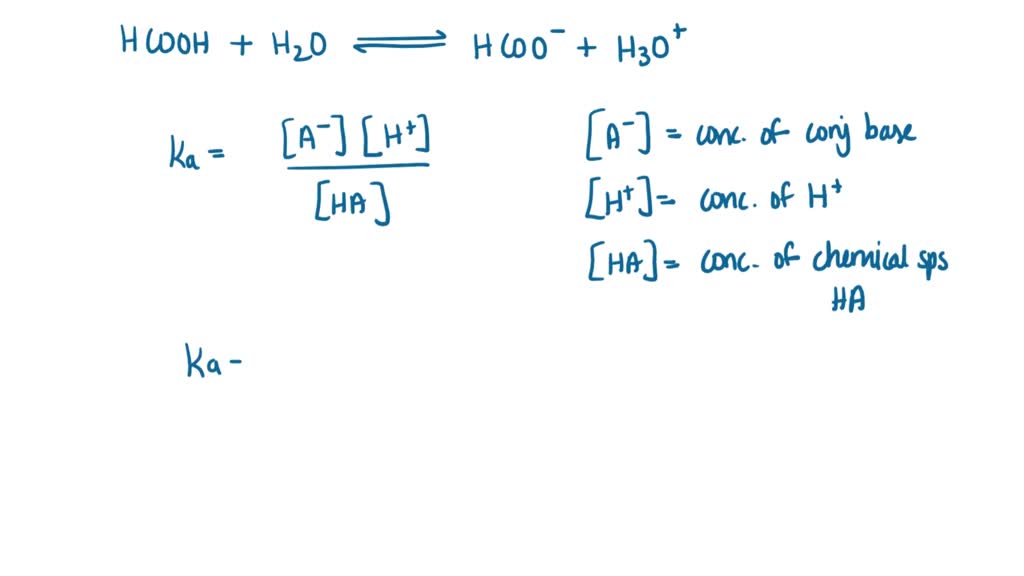
SOLVED: Write the acid dissociation constant expression, Ka, for the reaction below. HCO2H + H2O ⟷ HCO2- + H3O+

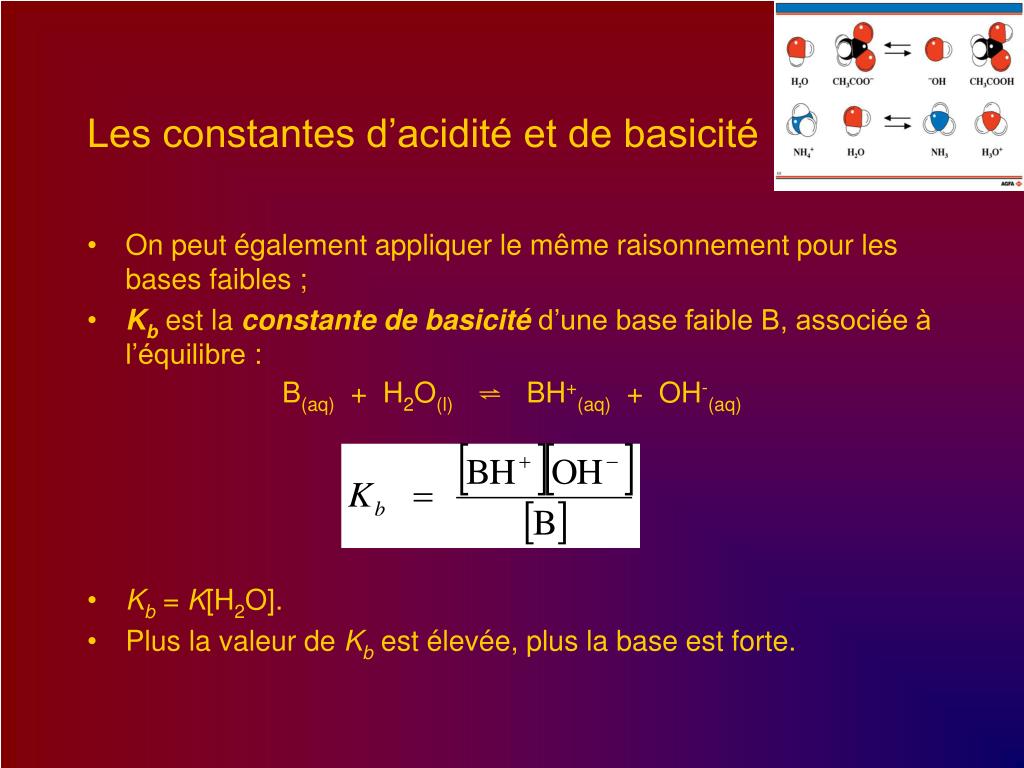
SOLVED: Ka: SO4^2- (aq) + H2O (l) ⇌ HSO4- (aq) + OH- (aq) Kb: HSO3- (aq) + H2O (l) ⇌ H2SO3 (aq) + OH- (aq)

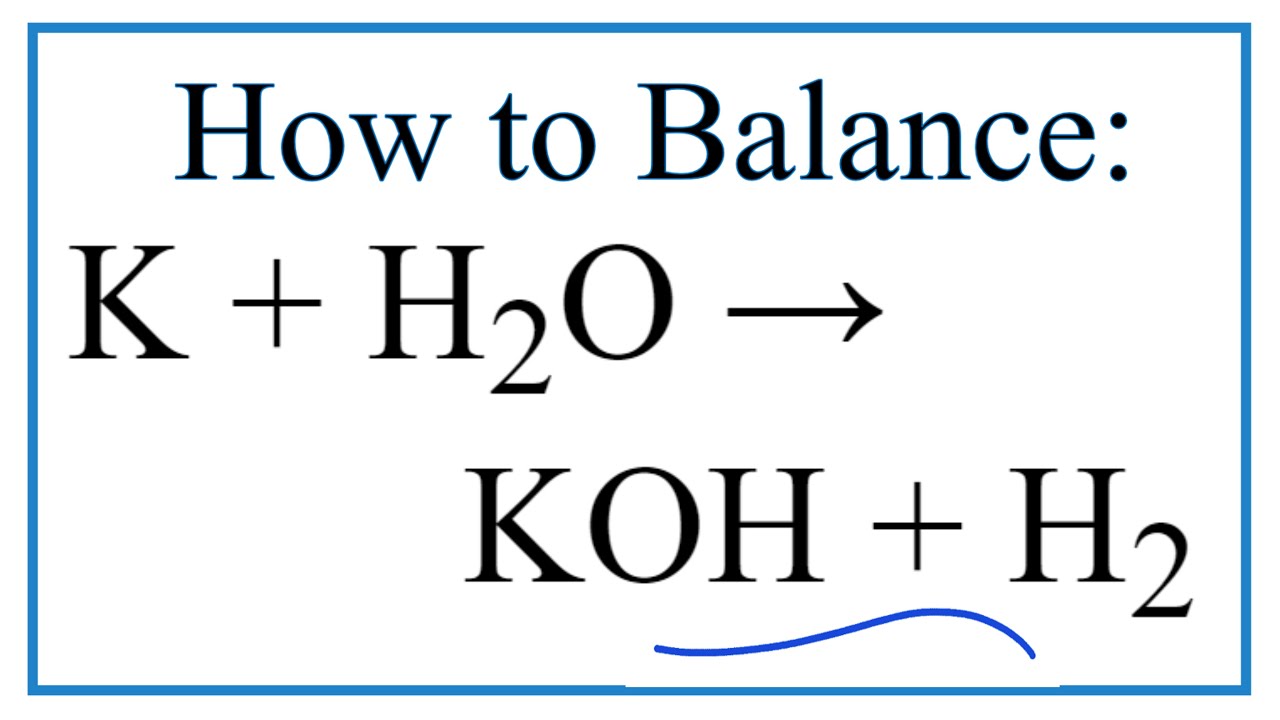
Oxidation Number method. K+H2O=KOH+H2. Balance the chemical equation by oxidation Number method. - YouTube

How to balance K+H2O=KOH+H2|Chemical equation K+H2O=KOH+H2|reaction balance K+H2O=KOH+H2| K+H2O= - YouTube