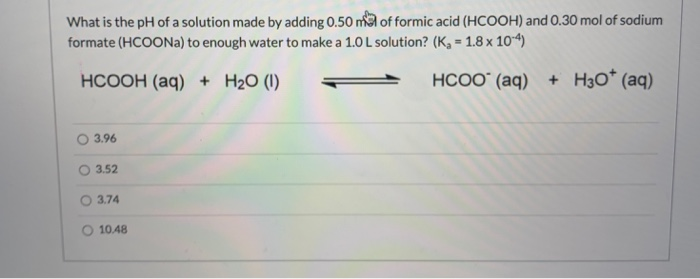
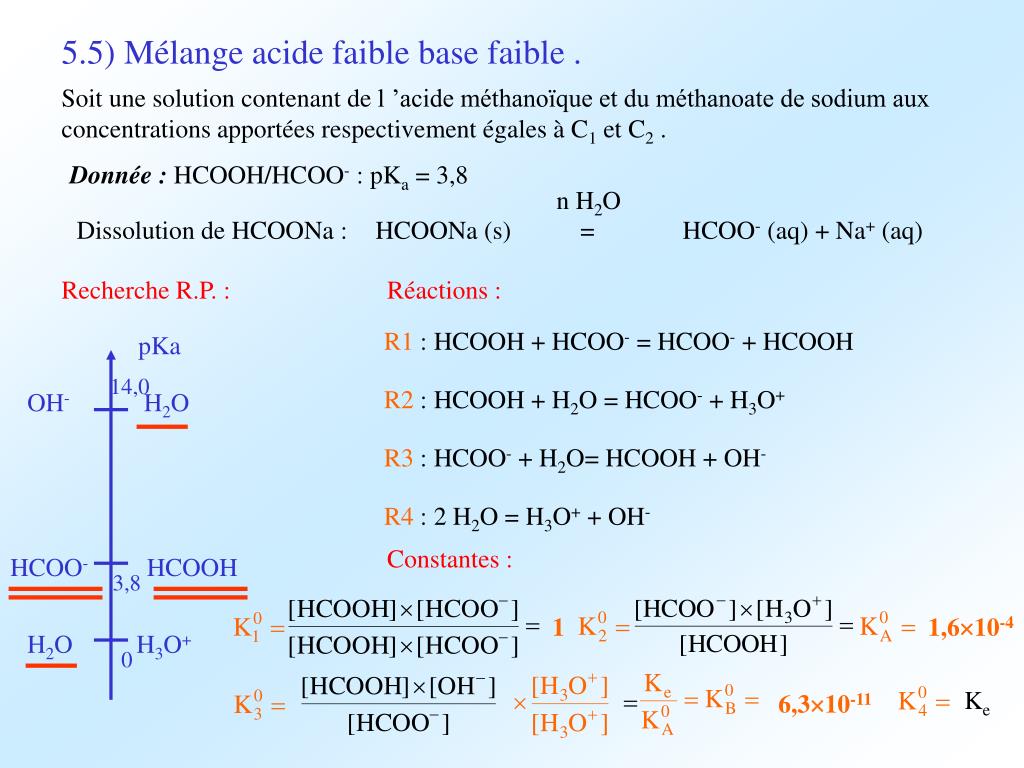
2 HCOONa + H2SO4 → 2 CO + 2 H2O + Na2SO4 A 0.964 gram sample of a mixture of sodium formate and sodium
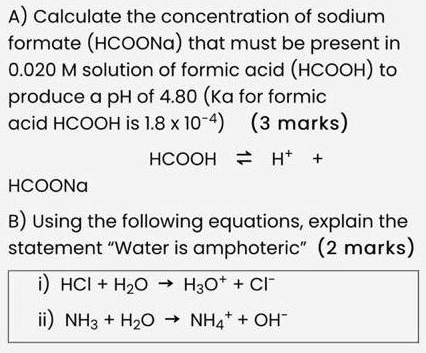
SOLVED: A) Calculate the concentration of sodium formate (HCOONa) that must be present in a 0.020 M solution of formic acid (HCOOH) to produce a pH of 4.80 (Ka for formic acid

SOLVED: The neutralization of formic acid with aqueous NaOH produces sodium formate (HCOONa) as the only product. The reaction can be represented as follows: HCOOH + NaOH -> HCOONa + H2O.
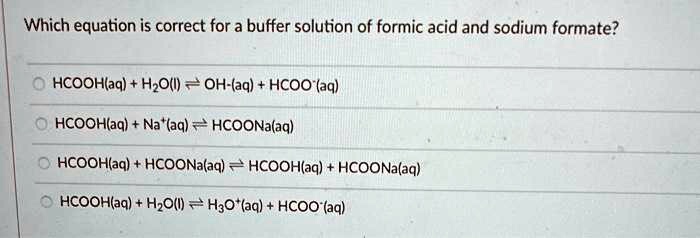
SOLVED: Which equation is correct for a buffer solution of formic acid and sodium formate? HCOOH(aq) + H2O(l) ⇌ H3O+(aq) + HCOO-(aq) HCOOH(aq) + NaOH(aq) ⇌ HCOONa(aq) + H2O(l) HCOOH(aq) + HCOONa(aq)

Reductive Hydrodehalogenation of Halogenated Carboxylic Acid Derivatives Using a DMSO/HCOONa·2H2O System | Organic Letters

Solved) - Which equation is correct for a buffer solution of formic acid and... (1 Answer) | Transtutors

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11

Über Das ternäre System HCOOHHCOONaH2O. Zur Kenntnis der sauren Natriumsalze der Ameisensäure - Elöd - 1927 - Zeitschrift für anorganische und allgemeine Chemie - Wiley Online Library

Find the equilibrium constant equilibrium HCOO + H2O + HCOOH + OH- In a solution of 0.1 M HCOONa. K (HCOOH) = 1.8 x 104 (1) 1.8 x 10-4 (275.56 10 (3) 5.56 x 10-11 (4) 1.8 x 10-18