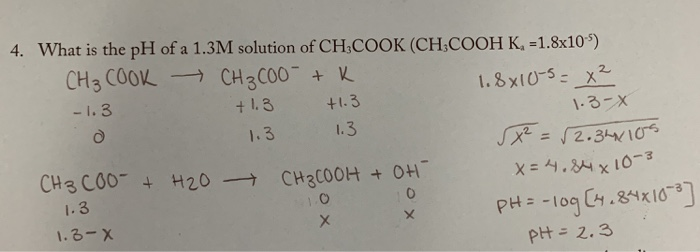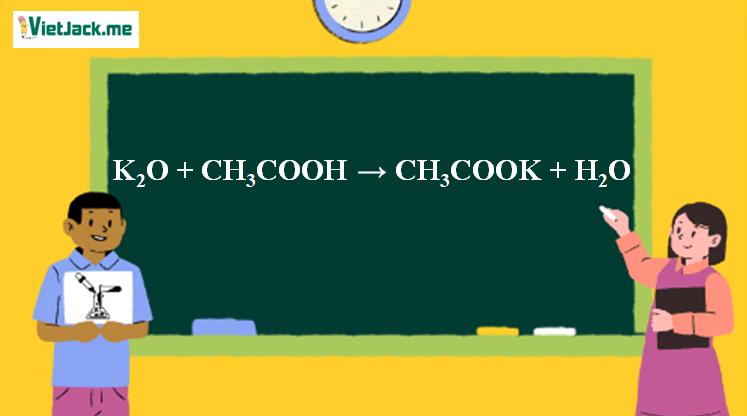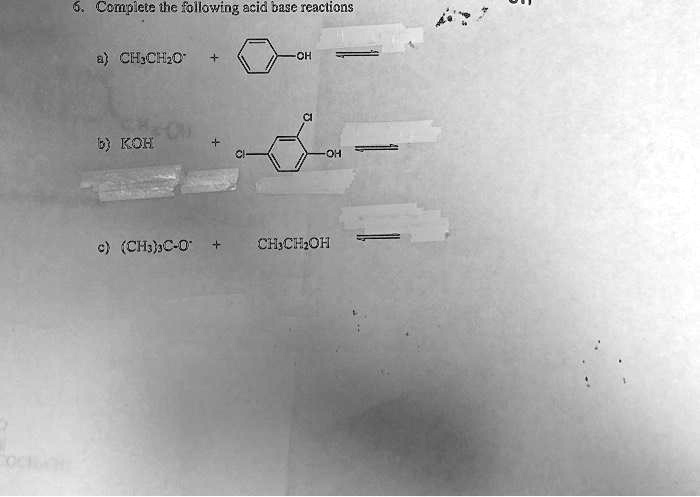
Acheter Acétate de Potassium CAS 127-08-2 ? - Acétate de potassium CAS 127-08-2 de qualité pharmaceutique sur Laboratoriumdiscounter.nl. Prix sympa et livraison rapide. Rabais de quantité!

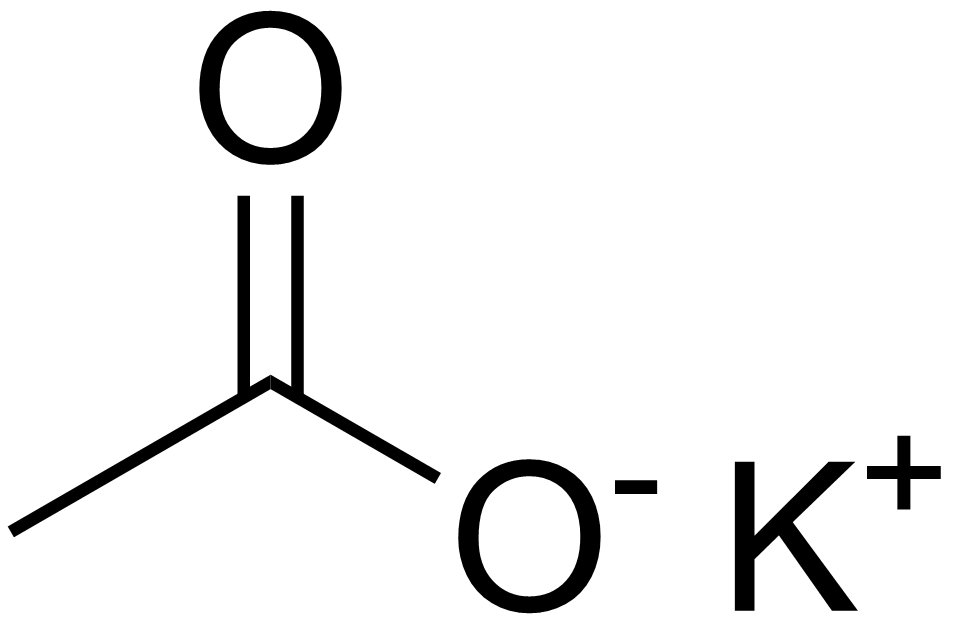
Potassium Acetate ACS Reagent - 99.0% Purity CAS No. 127-08-2 Chemical Formula CH3COOK | Procurenet Limited

Preparation of 3β-hydroxy-6-ethylchenocholane derivatives (subset B).:... | Download Scientific Diagram
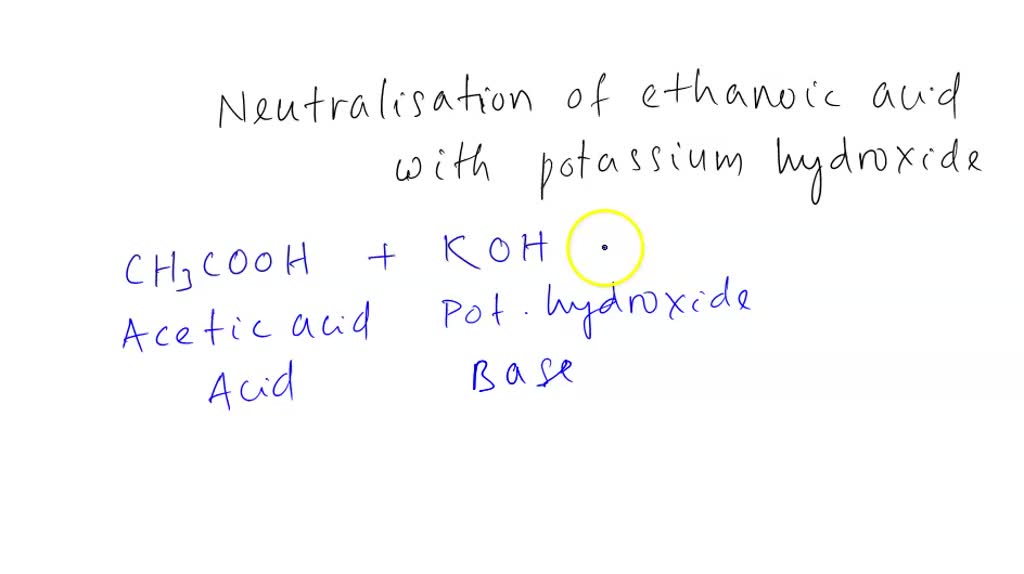
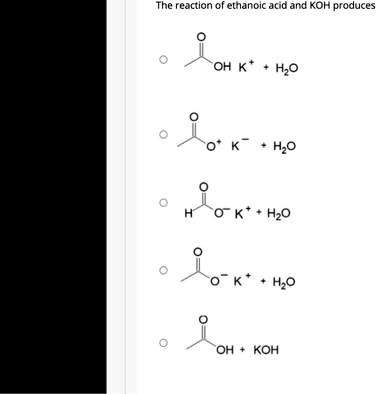
SOLVED: QUESTION 4 POINT Identify the correct chemical reaction for the neutralization of ethanoic acid by potassium hydroxide. Select the correct answer below: CH3COOH + KOH â†' CH3COOK + H2O CH3COOH +
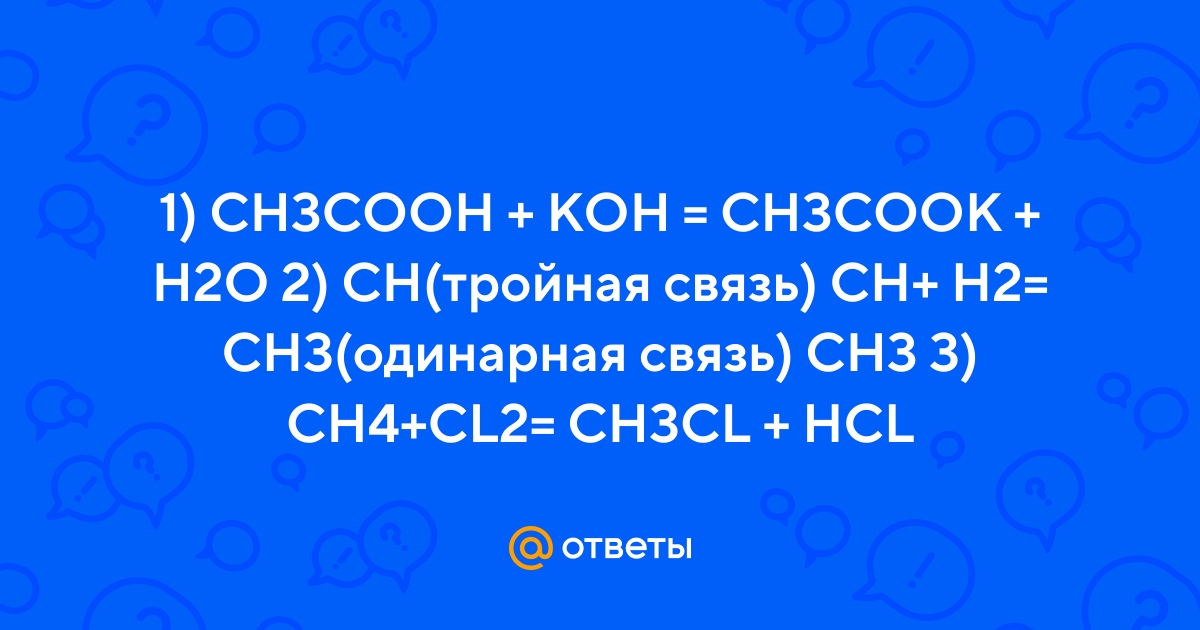
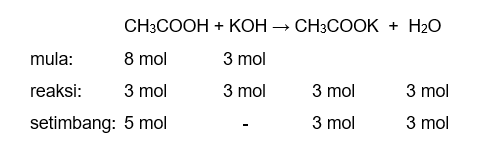
Ответы Mail.ru: 1) CH3COOH + KOH = CH3COOK + H2O 2) CH(тройная связь) CH+ H2= CH3(одинарная связь) CH3 3) CH4+CL2= CH3CL + HCL
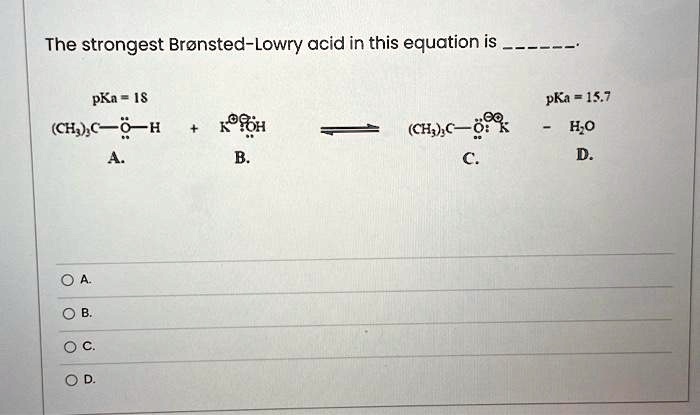
SOLVED: Texts: The strongest Brønsted-Lowry acid in this equation is pKa = 1S pKa = 15.7 - HOD (CH3COOH + KOH → CH3COOK + H2O) (CH3COOH + KOH → CH3COOK + H2O) OA. OB. OC. OD.
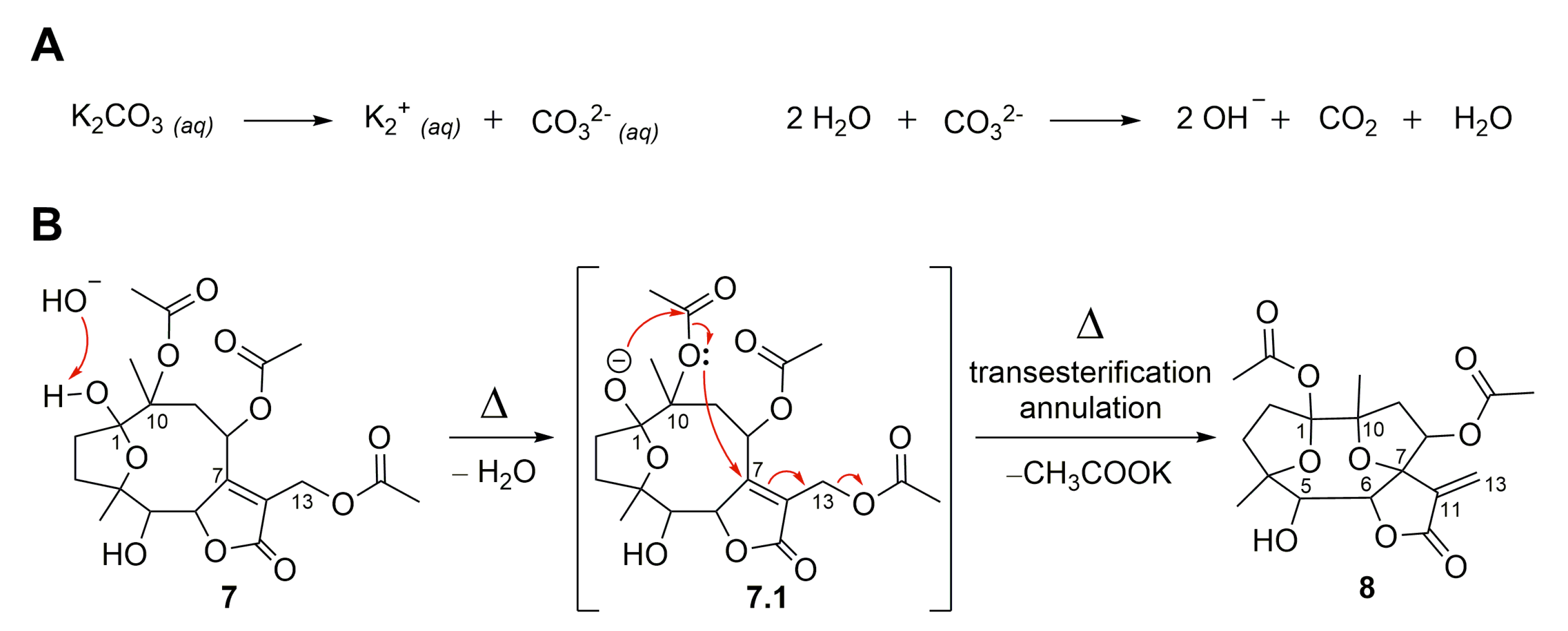
Molecules | Free Full-Text | Semisynthetic Sesquiterpene Lactones Generated by the Sensibility of Glaucolide B to Lewis and Brønsted–Lowry Acids and Bases: Cytotoxicity and Anti-Inflammatory Activities