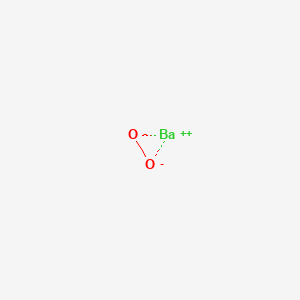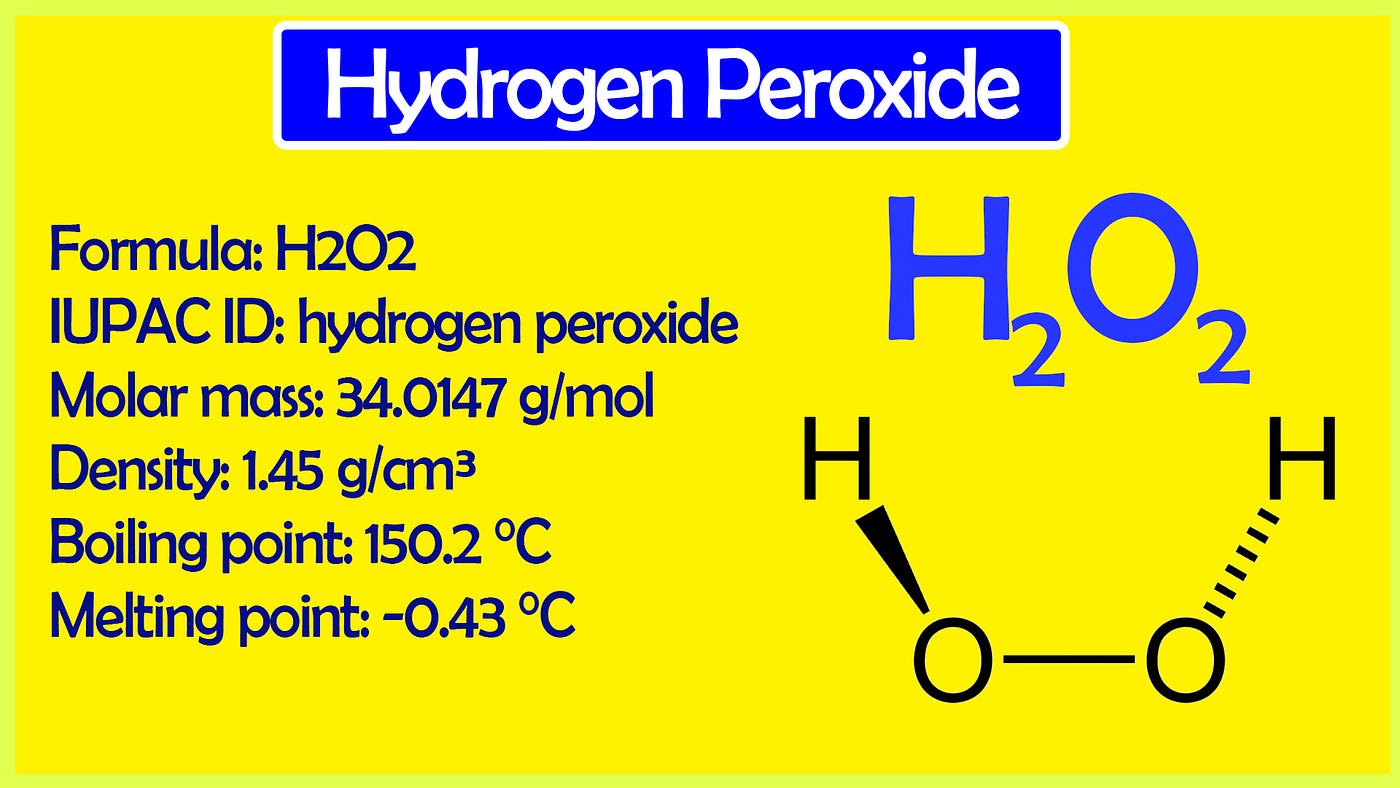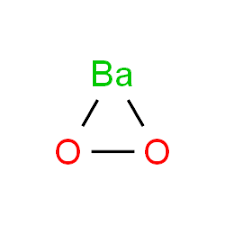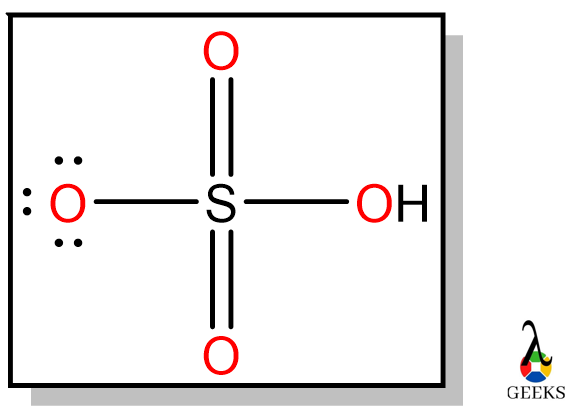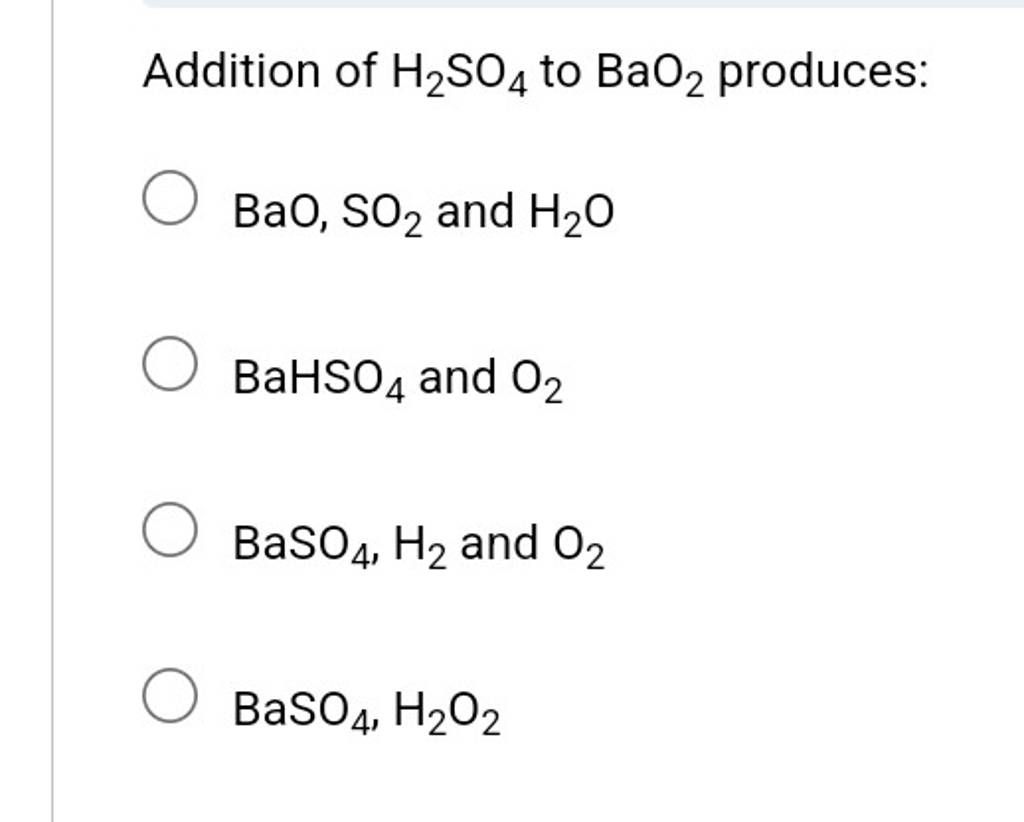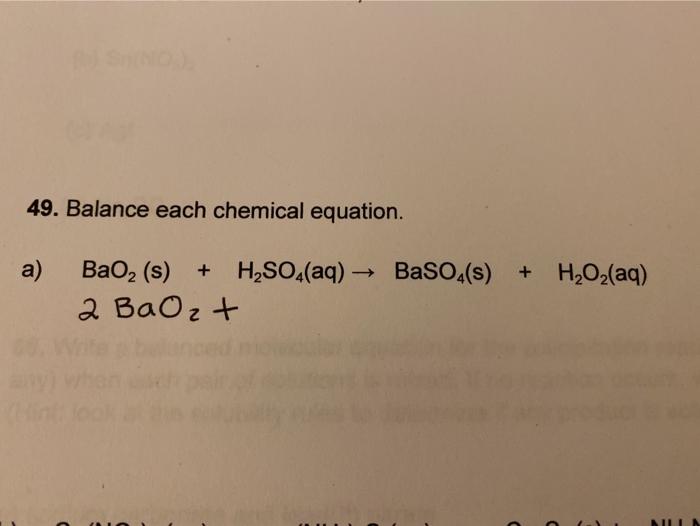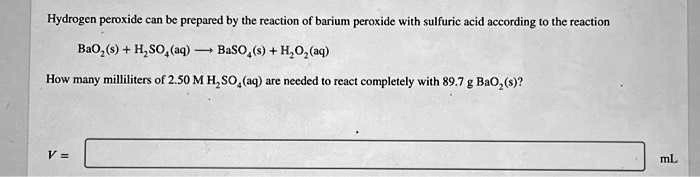
SOLVED: Hydrogen peroxide can be prepared by the reaction of barium peroxide with sulfuric acid according to the reaction BaO2 + H2SO4 → BaSO4 + H2O. How many milliliters of 2.50 M

Achetez en gros Peroxyde De Baryum Cas: 1304-29-6 Chine et Peroxyde De Baryum à 2 USD | Global Sources
General and inorganic chemistry I: Required knowledge This document describes the scope of knowledge that students are expected

SOLVED: Hydrogen peroxide can be prepared by the reaction of barium peroxide with sulfuric acid according to the reaction: BaO2(s) + H2SO4(aq) â†' BaSO4(s) + H2O(aq) How many milliliters of 2.00 M

Chemical Reactions: evidence, writing them, balancing them Zumdahl Chapter 6 Notes Start Date: ______. - ppt download
What is the oxidation state of the most electronegative element in the products of the reaction between BaO2 and dil.H2SO4? - Quora