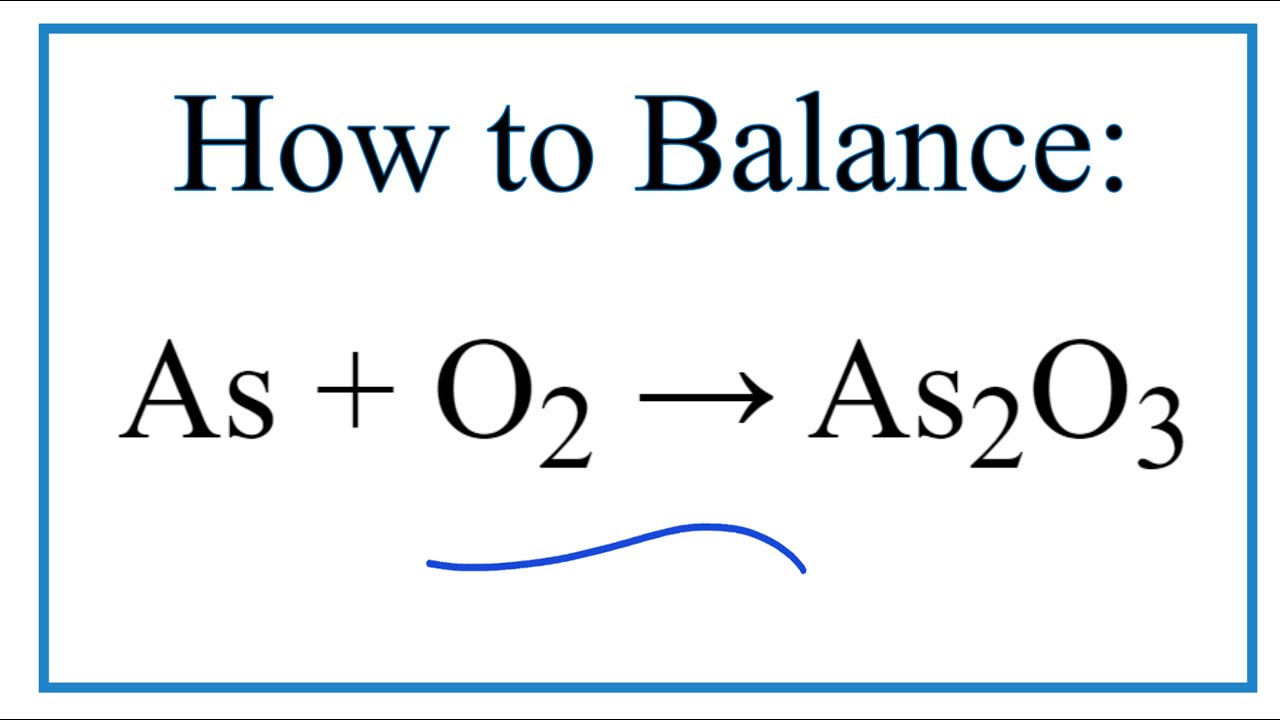
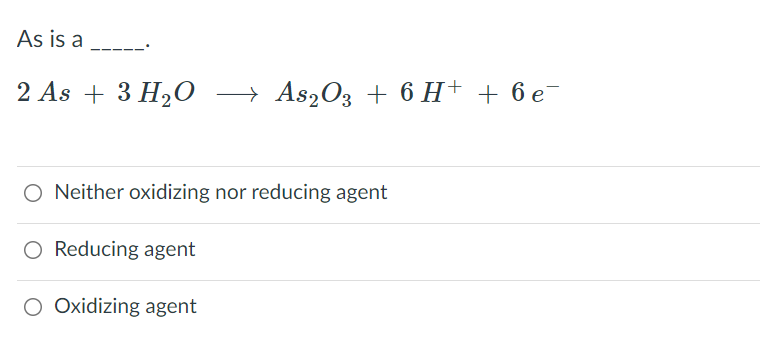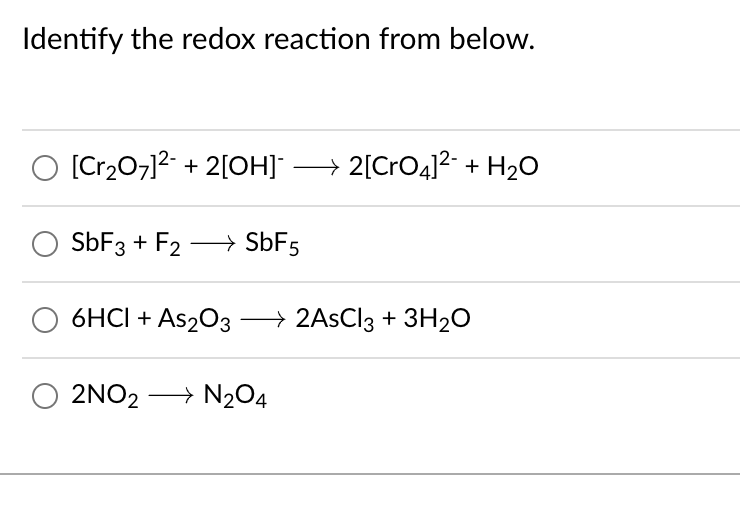Which one of the following reactions involves disproportionation? 2H2SO4 + Cu → CuSO4 + 2H2O + SO2 As2O3 + 3H2S – As2S3 + 3H20 2KOH + Cl2 - KCI + KOCI + H20 Ca3P2 + 6H20 – 3Ca(OH)2 + 2PH3

SOLVED: A solution of iodine was standardized by titration with H3AsO3 prepared from weighing and dissolving 141.6 mg of pure As2O3. The titration reaction is H3AsO3 + I2 + H2O = H3AsO4 +

SOLVED: 7-12. Arsenic(III) oxide (As2O3) is available in pure form and is a useful (but carcinogenic) primary standard for oxidizing agents such as MnO4. The As2O3 is dissolved in base and then

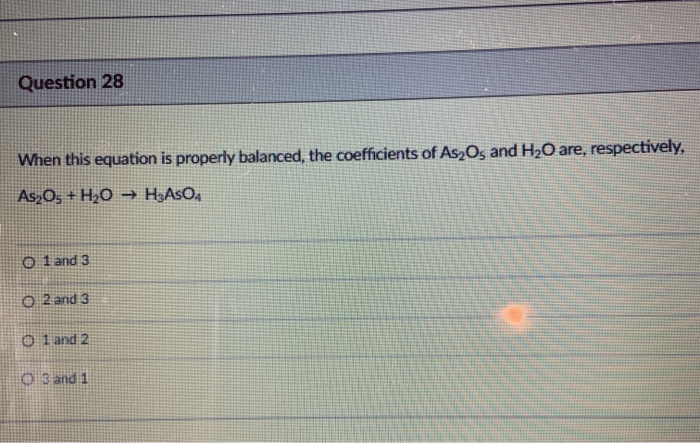
SOLVED: When this equation is properly balanced, the coefficients of As2O3 and H2O are, respectively, As2O3 + H2O â†' HAsO3 + ? 0l4 and ? 0l2 and ? Oland 2 Ol3ald 1

SOLVED: Balance the following redox equation in acidic solution As2O3 (s) + NO3-(aq) –> H3AsO4 (aq) + N2O3 (aq) What is the coefficient in front of As2O3 in the balanced equation? P.S.
Which of the following reactions involves disproportionation reaction 2H2SO4+Cu—CuSO4+2H2O+SO2 As2O3+3H2S—As2S3+3H2O 2KOH+Cl2—KCl+KOCl+H2O Ca3P2+6H2O—Ca(OH)2+2PH3
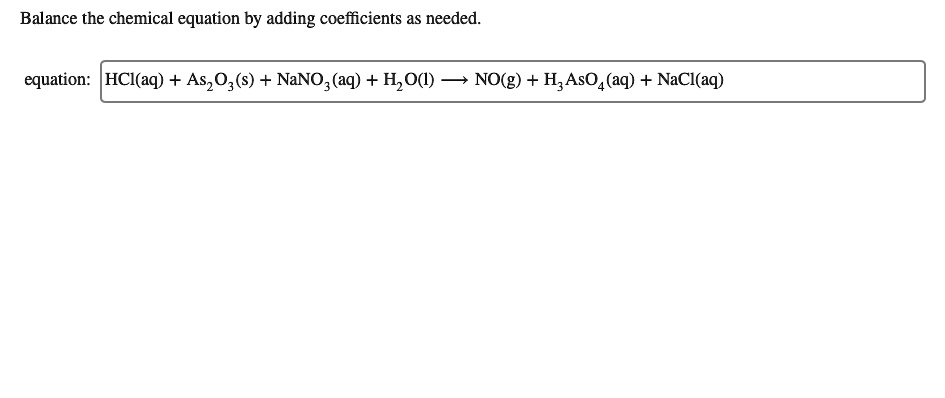
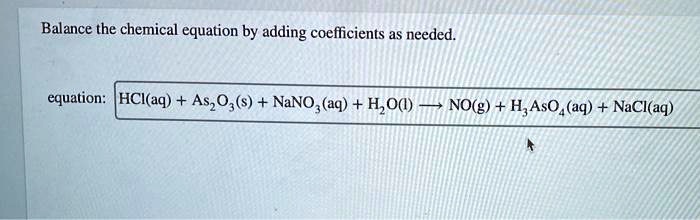
SOLVED: Balance the chemical equation by adding coefficients as needed. Equation: HCl(aq) + As2O3(s) + NaNO2(aq) + H2O(l) â†' NO(g) + H3AsO4(aq) + NaCl(aq)